Clinical Research IO’s Survey Finds That Coronavirus is Disrupting Clinical Research Sites

Clinical Research IO‘s recent web survey found that COVID-19 is disrupting clinical research sites worldwide. The results are indicative of a significant slowdown in new drug development and long term effects on healthcare in general.
- 25% of sites have stopped recruiting
- Of the 75% that are continuing enrollment, about half (37%) are considering halting new patient enrollment
- Sites that are still enrolling report on average, a 27% drop in recruitment
This means that aggregate recruiting is down by approximately 50% and studies will need double the time to fill enrollment. If half of the 75% stop recruiting, then clinical trial enrollment timelines will increase by four times.
Among the sites that are still conducting study visits, challenges remain.
- Patient retention has started to fall, with an average decline of 9%,
- The number of protocol deviations have risen, with an average increase of 4%
- Nearly two thirds of sites (63%) have prohibited on site monitoring visits, thus inhibiting effective oversight by sponsors
- These values are expected to increase with ongoing travel limitations and quarantines
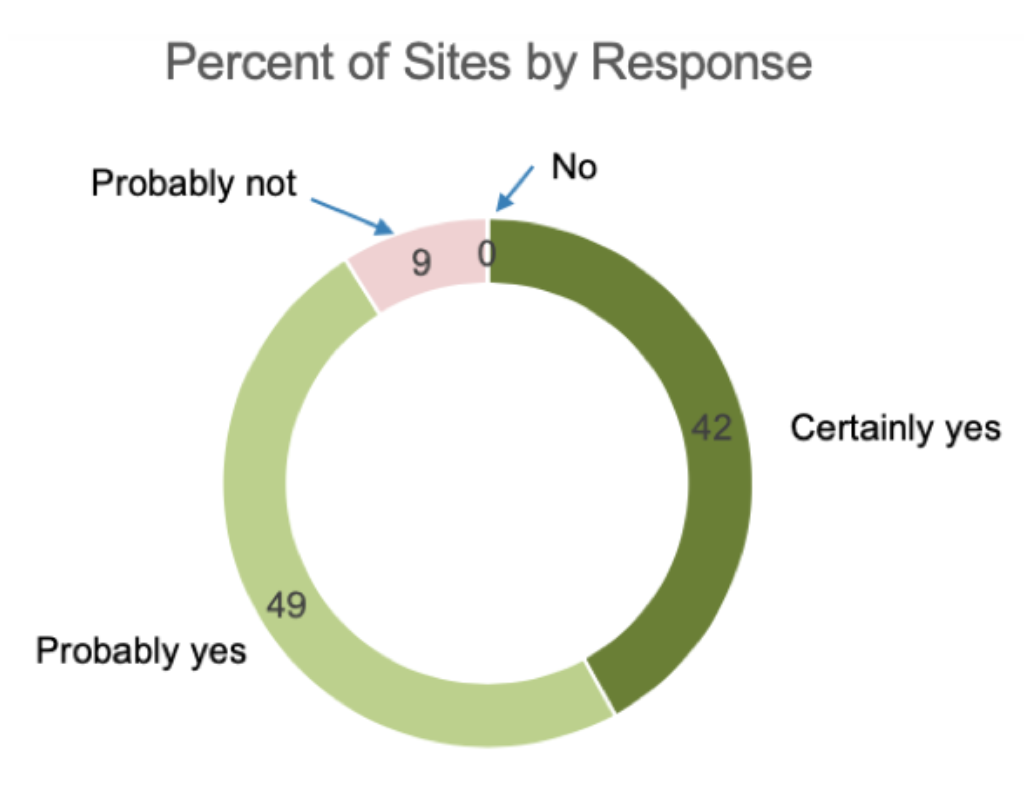
In addition to identifying declining enrollment and retention rates, the survey revealed that over 90% of sites would utilize sponsor provided technology, meaning a direct electronic data capture tool combined with a document upload feature.
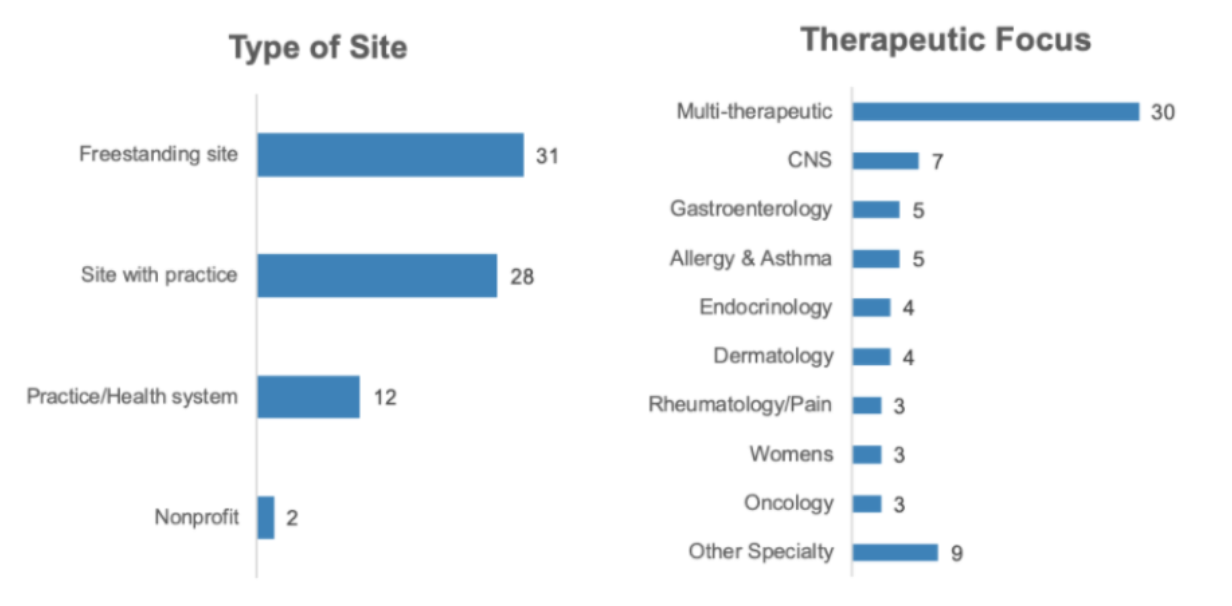
The web survey was fielded from March 19-23, 2020. A total of 73 sites across different therapeutic focuses participated.