Built for Sites, Better for Your Trials. Clearly CRIO
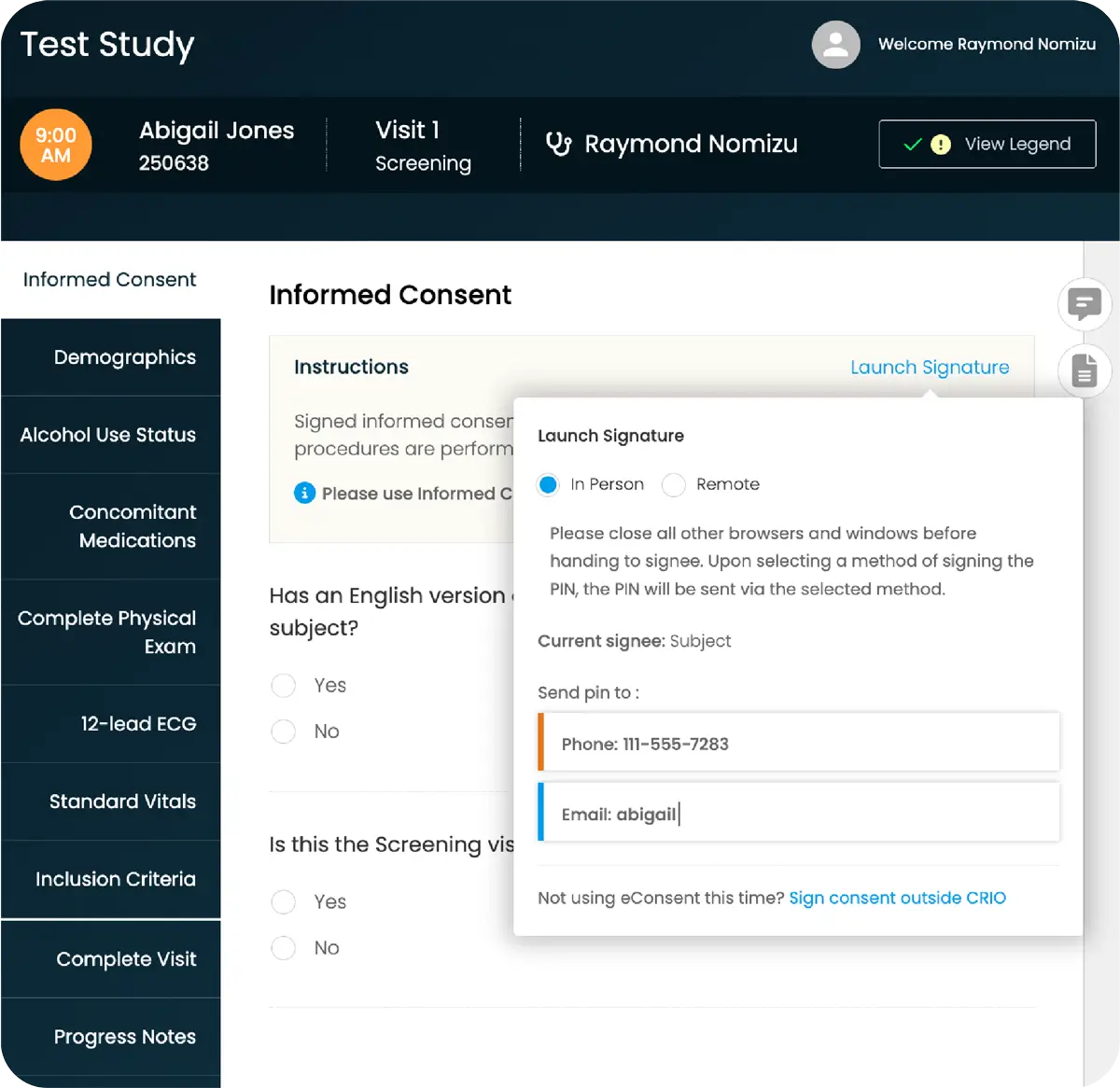
Intuitive eSource Enabling Faster, Smarter Decisions
You need reliable, protocol-driven data, but sites can’t focus on quality when bogged down with administrative details and manual tasks. There’s an eSource solution that makes it all easier.
It’s clearly CRIO.

Your eSource Solution
CRIO is an intuitive eSource solution that collects data directly at the point of patient interaction to lighten site burden while driving protocol compliance. Created by a team of clinical research experts who understand what sites need to perform better, it’s built to make trials run seamlessly from the start:
-
Fully configurable and scalable templates standardize data capture
-
Real-time data access enables remote monitoring and collaboration
-
Easily accessible reporting and query capabilities enhance data quality and site performance
-
A connected ecosystem supporting essential data collection activities streamlines workflows
See CRIO in Action
Find Out What CRIO Can Do for You
-
40% Higher Enrollment
-
40% Faster Startup
-
40% Fewer Protocol Deviations
