eRegulatory
Electronic regulatory binder
Streamline regulatory workflows with paperless binders and the first-ever digital delegation log
Manage training documents
Instead of filing the same GCP, IATA and other common files multiple times, site users can file them once for the site or themselves, and then let CRIO file into each study automatically. Expiration alerts and versioning ensure users keep their credentials up to date.
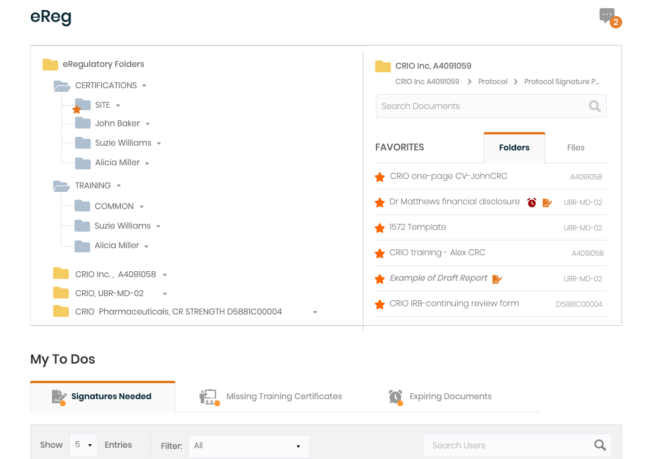
Manage study ISF binders digitally
Upload, version, route, and e-sign regulatory documents. Configure study folders and save favorite folder structures as templates. Track expiring documents, bulk sign documents, and view/respond to CRA queries.
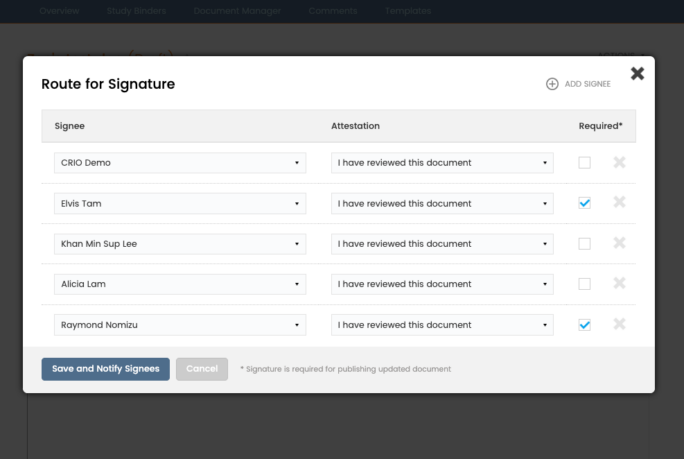
Digitize the Delegation Log
Study teams can build electronic delegation logs. Copy duty profiles from one user to the other. Easily keep track of requested and delegated duties. Each user can view their own personalized delegation log with one click. CRAs love our downloadable, print-friendly DOA log with legible audit trails!
Solutions
Join the industry’s leader in electronic source data capture for clinical trial sites. CRIO eSource is a user-friendly, web-based tool to capture site source data in real-time.
Discover eSourceCRIO Publisher allows sponsors, networks and other users running the same study at multiple sites to build, version and push the templates to the sites on the trial.
Discover PublisherCRIO Reviewer allows sponsors and CROs to review eSource data as it's saved, and to query, lock, medical code and extract the data. It's a remote monitoring and EDC tool rolled into one.
Discover Reviewer EDCCRIO’s eConsent automates the completion and execution of the Informed Consent, and is built right into CRIO’s eSource workflow.
Discover eConsentWith CRIO’s eRegulatory, research sites can streamline regulatory workflows with paperless binders and an electronic delegation log.
Discover eRegulatoryCRIO’s innovative partnership with Pluto Health Health delivers electronic medical records, alongside clinical care insights, straight to the investigator, within minutes! PIs can review health data and insights, and annotate the data before entry into source and the EDC.
Discover Medical Record APICRIO's reporting module lets users filter, sort, search, download, schedule and export data. Users can also have their own Business Intelligence tool query their CRIO data directly.
Discover ReportingCRIO’s site-based CTMS lets clinical research sites run their "back office operations", including patient recruiting, scheduling, financial management and more.
Discover Site CTMSSites can issue and load patient stipend cards with preset or manual amounts. Our reports keep track of how much you’ve paid each subject, enabling easy 1099 reporting.
Discover Patient Stipends