Products Built for Sites & Better for Trials
Whether you need integrated eSource technology, user-friendly clinical trial management software, or a paperless regulatory management system, the eClinical solution that’s the cornerstone of your work is clearly CRIO.
Find your perfect products now.
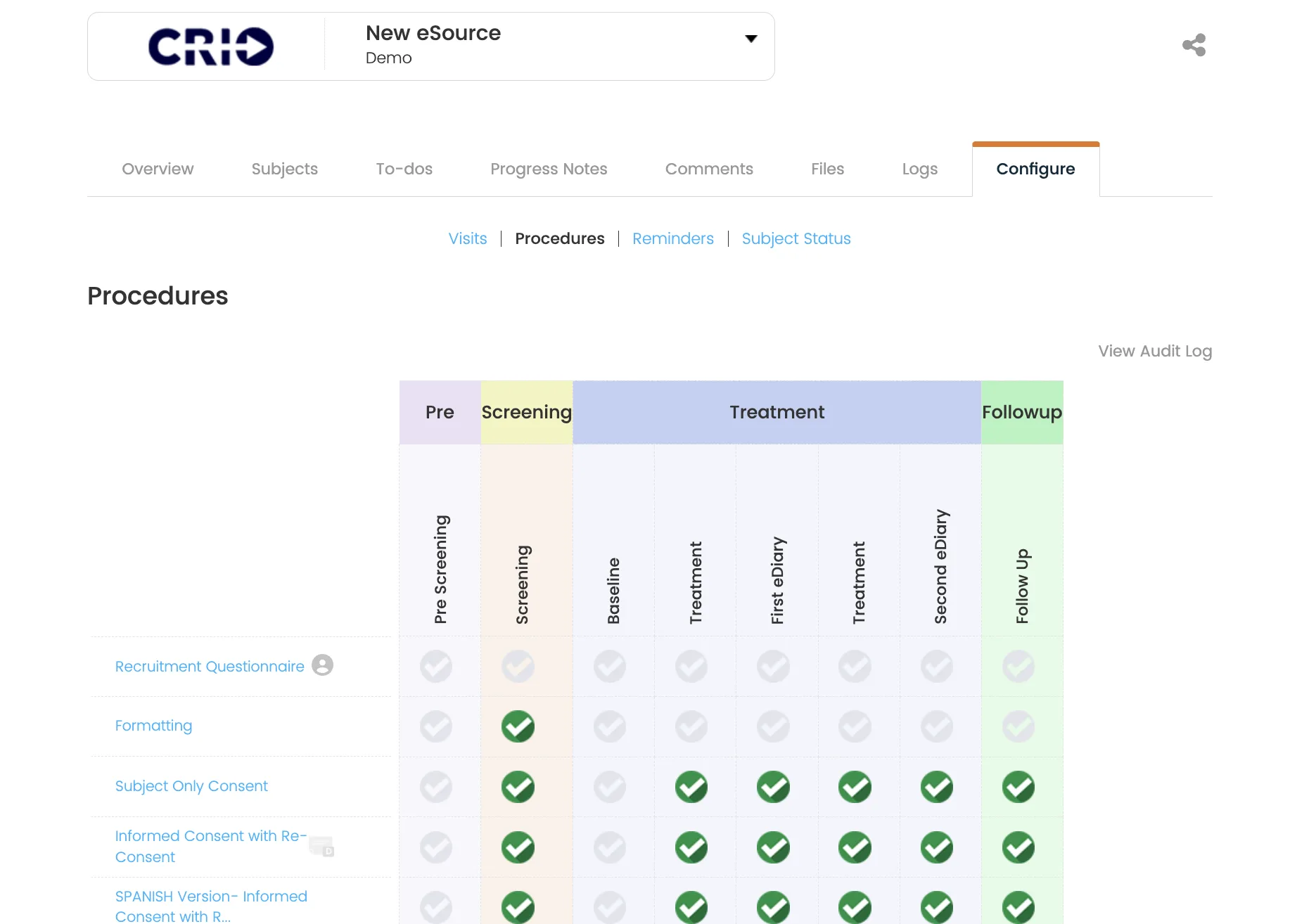
Real-time visibility for better data and better site performance.
Higher-performing sites for more accuracy and efficiency.
End-to-end technology platform for streamlined operations.

With 40% higher enrollment, 40% faster startup, and 40% fewer protocol deviations, why wouldn’t you use CRIO’s eClinical solutions?