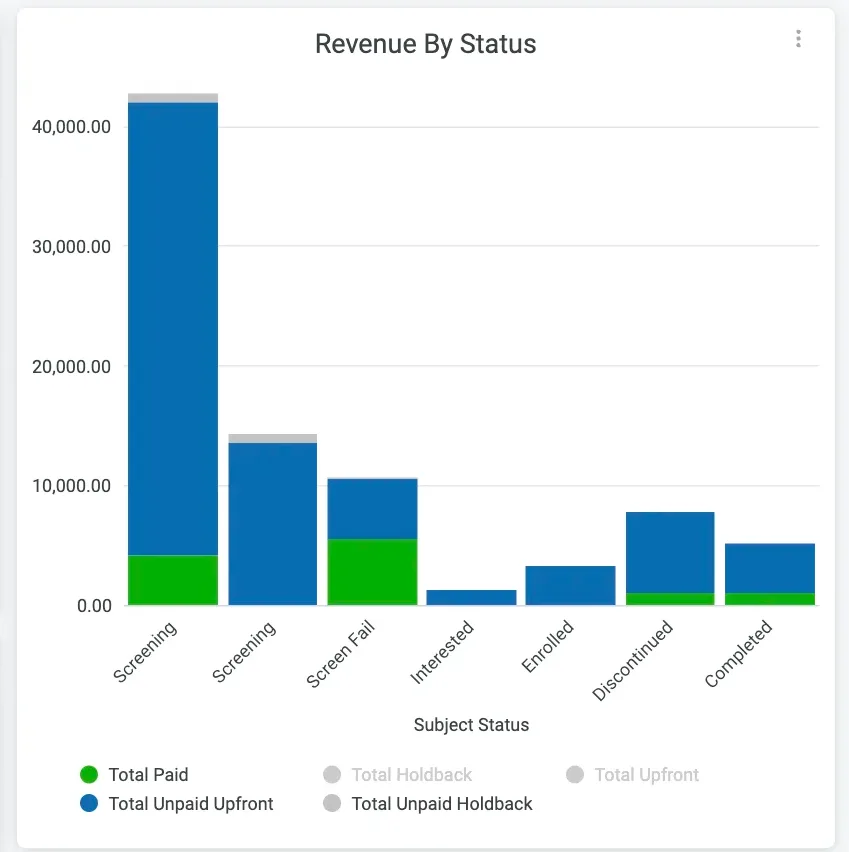
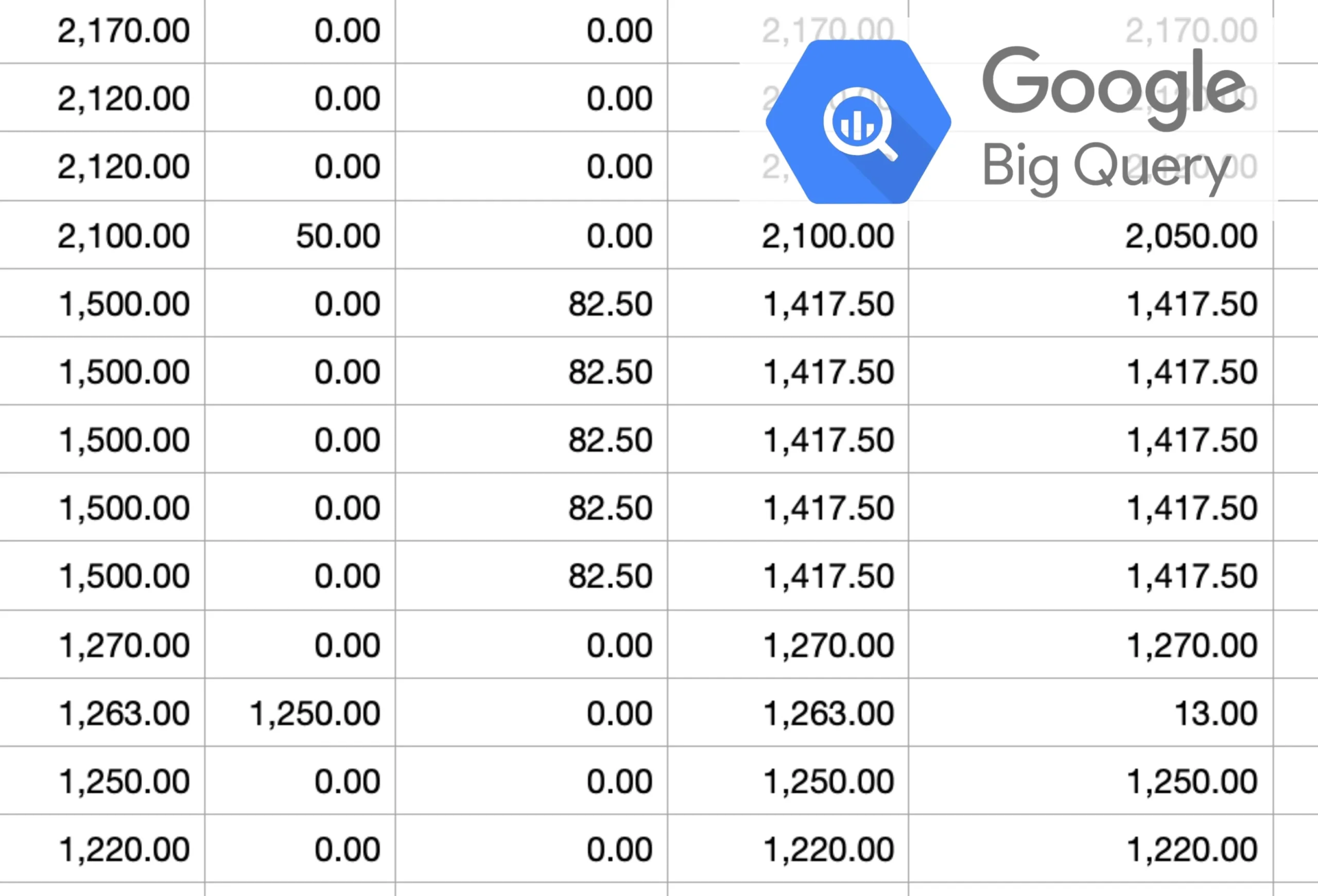
The solution that uses real-time, customizable, and easily accessible reporting and query capabilities to optimize site operations, data quality, and remote monitoring is clearly CRIO.

Access Your Data How You Want and When You Want
Reporting is fully integrated with the entire CRIO platform — including our site CTMS, eRegulatory, Reviewer EDC, and eSource solutions — so you can report on nearly every data point in real time.
Standard and Custom Reporting Options
- Create custom reports, then preschedule, download, and export as needed with CRIO’s built-in reporting tools
- Custom report design service is also available
Direct Data Access for Business Intelligence Tools
- Use your business intelligence tools to directly access data within the platform
- Copy or query data from CRIO’s BigQuery replica database with only 15 minutes latency