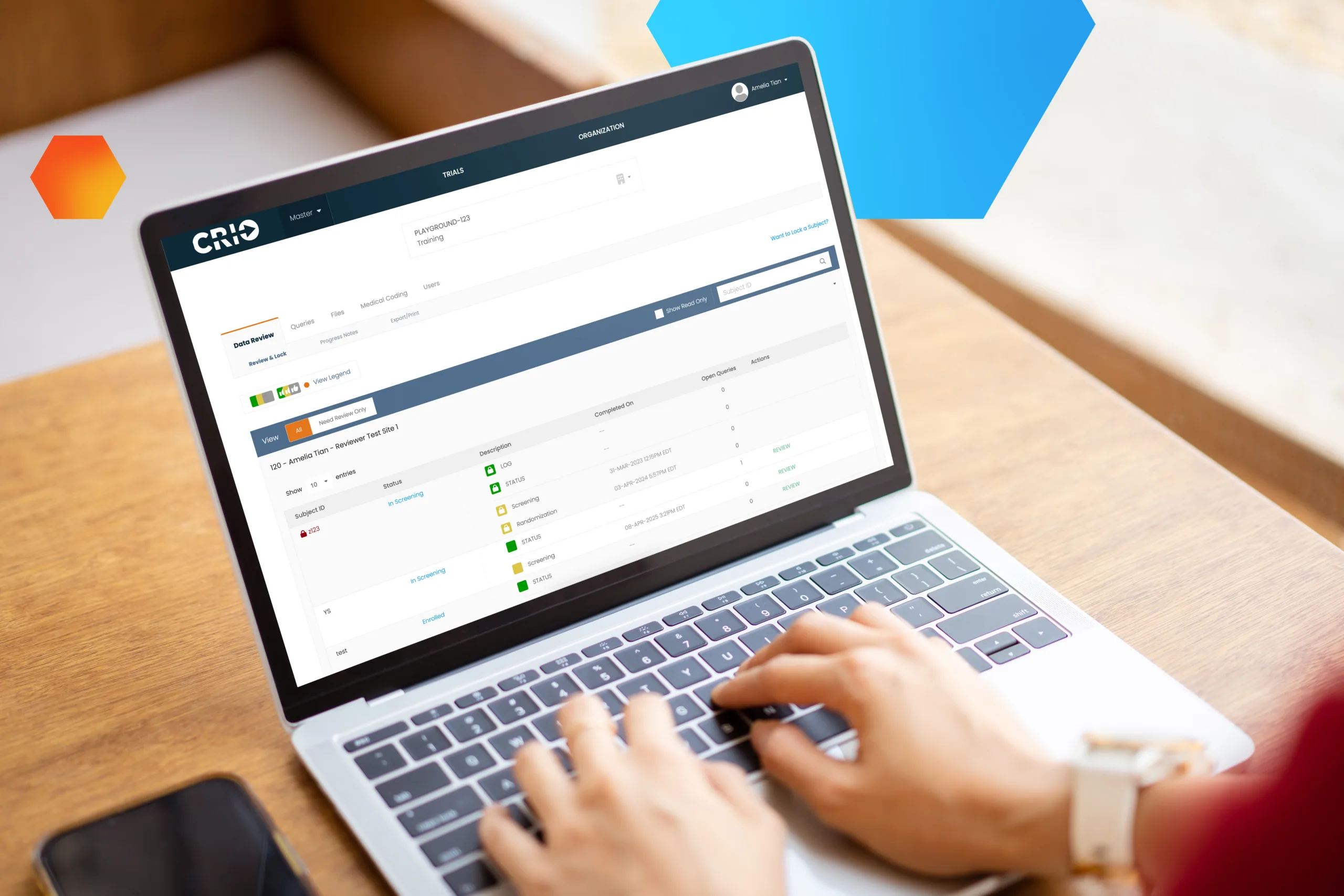
Empower Your Sites to Improve Performance and Data Quality
Visibility into clinical trial data enables the right choices at critical moments. But how can sites focus on quality when they’re bogged down with administrative work? Luckily, there’s a Central eSource that automates your protocol, embeds quality at the point of capture, and enables fully remote monitoring to improve site performance and deliver better, faster data. It’s clearly CRIO.
-
40% higher enrollment
-
40% faster site startup
-
90% monitoring productivity gain
Your Central eSource for Faster, Smarter Decisions
CRIO’s Central eSource technology delivers tremendous site operational efficiency and unmatched clinical data accuracy.
Leverage Existing Site Technology
Many high-performing sites already use CRIO. Central eSource allows you to leverage these benefits across your entire trial at every site.
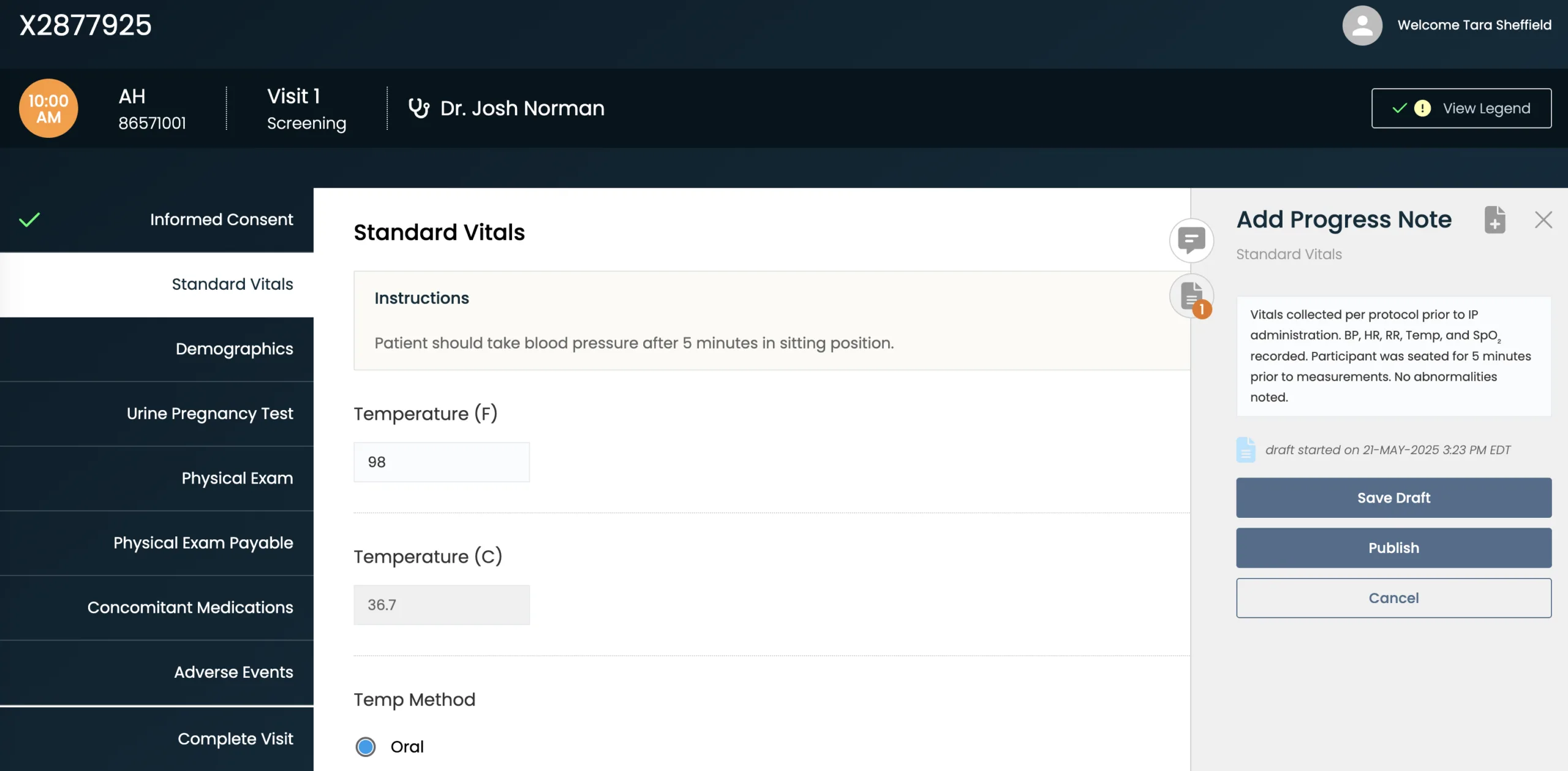
Build Compliance at the Source
Central eSource templates — configured and automated to your protocol — incorporate real-time alerts and run logic checks to ensure reliable endpoint data.

CRIO-powered sites out-enroll others by 40% and have 2x the patient diversity.

CRIO-powered studies reduce costs by 50%.
Review Data Immediately and Continuously
A centralized, fully remote, and continuous review process captures deviations early and delivers insights sooner.
Empower Your Sites
Central eSource and site configurability reduce your site’s administrative burden, improving enrollment, accelerating startup, and freeing them to focus on patients.
Get EDC Data Faster With Fewer Queries
Configure your Central eSource template to match your eCRF, integrate with leading EDC vendors, and enable sites to automate data transmission.
Save Big
Real-time source data access and seamless EDC integration — with our EDC solution or with top vendors — substantially decrease on-site monitoring time, reduce source data verification, and cut travel costs.
Your Central eSource Solution
Built for Sites & Better for Your Trials

Trial Tested, Site Approved
Don’t force tech on your sites. Get more out of the eSource solution they’re already using: CRIO.
Contact our team to find out how sites on your studies are already using CRIO.
Go Further, Faster With CRIO
Our eSource software solution seamlessly integrates with leading eClinical technology vendors, further enhancing your clinical trial.