In the world of clinical trials, the question isn’t if you’ll face an inspection—it’s when. Whether you’re a site, sponsor, or CRO, maintaining inspection readiness isn’t just about compliance; it’s about protecting participant safety and ensuring data integrity. Let’s explore what it takes to confidently answer “yes” when that knock on the door comes.
What is Inspection Readiness?
Inspection readiness is an ongoing state of preparedness that ensures adherence to regulatory frameworks including ICH-GCP, FDA regulations (21 CFR Parts 11, 50, 54, and 312), EU Clinical Trials Regulation (CTR) No 536/2014, GDPR, MHRA GCP requirements, WHO GCP guidelines, and other applicable standards. It’s the foundation that protects participant safety and well-being while maintaining compliance with study protocols.
The stakes are high. According to FDA inspection data from 2024, protocol non-compliance accounts for 55.4% of investigator citations, while inadequate case history records represent 24.1% of findings. These statistics underscore a critical truth: being prepared isn’t just good practice—it’s essential for maintaining data integrity and avoiding costly delays.
Understanding the Inspection Process
While inspection procedures vary by regulatory authority, several key elements remain consistent:
- Formal notification from the regulatory authority announcing the upcoming inspection
- Opening meeting to establish scope and set clear expectations
- Document requests made before, during, or after the inspection
- System access requests ranging from over-the-shoulder viewing to direct access through dedicated inspector accounts
Understanding this process helps organizations prepare systematically rather than scrambling when notification arrives.
The Technology Advantage: Why Electronic Systems Matter
The days of rifling through filing cabinets and binders during inspections are numbered. Electronic systems have transformed inspection readiness from a logistical nightmare into a streamlined process.
Consider the practical difference: An inspector asks for all adverse event reports from the past six months. With paper systems, this means searching through multiple binders, potentially missing documents, and consuming valuable inspection time. With an electronic system like CRIO, it’s a matter of clicks—instant, complete, and auditable.
Electronic systems deliver clear advantages:
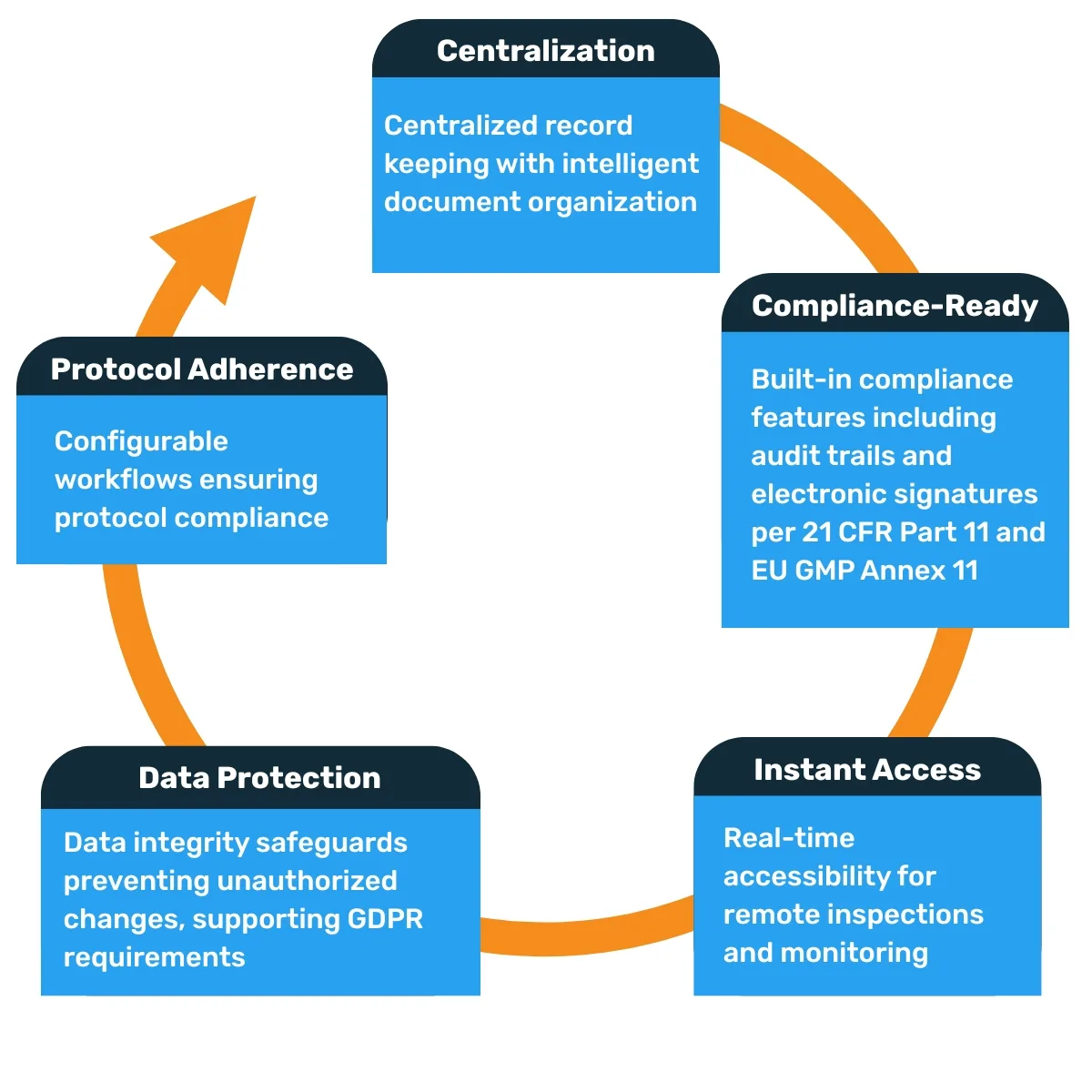
Embracing the Regulatory Evolution
The regulatory landscape has evolved dramatically, particularly following the pandemic. The FDA’s Remote Regulatory Assessment (RRA) guidance and ICH E6(R3) demonstrate regulators’ embrace of technology and risk-based approaches.
Key principles from ICH E6(R3) that support technology adoption:
- Media neutrality: Choose the electronic system that works best for your organization
- Flexibility and innovation: Leverage wearables, remote monitoring, and novel approaches
- Proactive quality: Build quality into processes from the start
- Data governance: Ensure responsible data collection, storage, and access in compliance with GDPR (where applicable)
- Risk proportionality: Balance oversight with operational efficiency
As Jason Wakelin-Smith from MHRA recently noted: “It is time for sponsors to adopt risk proportionality knowing that there is support from the regulators for its use.”
The Nine Pillars of Inspection Readiness
True inspection readiness requires excellence across nine critical areas:
1. Regulatory Compliance
Maintain adherence to FDA regulations (21 CFR Parts 11, 50, 54, and 312), EMA guidelines, MHRA GCP requirements, WHO GCP Guidelines ICH-GCP, and other applicable frameworks. This isn’t just about knowing the regulations—it’s about demonstrating consistent application.
2. Study Documentation
Keep complete, accurate, and current records including investigator site files (ISF), source documents, and CRFs in accordance to with 21 CFR Part 312 requirements. Documentation should tell the complete story of your trial.
3. Training and Delegation
Ensure all staff are properly trained with documented records and clear delegation logs per 21 CFR Part 312. Every team member should understand their responsibilities and limitations.
4. Participant Safety and Rights
Implement robust informed consent processes in compliance with 21 CFR Part 50 and maintain systems that prioritize participant safety above all else. Ensure proper documentation of financial interests per 21 CFR Part 54.
5. Investigational Product Management
Maintain secure storage with appropriate temperature controls and meticulous accountability logs. Every dose should be traceable from receipt to disposition in accordance with 21 CFR Part 312 requirements.
6. Data Integrity
Ensure accurate and timely data entry, prompt query resolution, and comprehensive audit trails meeting 21 CFR Part 11 and EU GMP Annex 11 standards. Data should be ALCOA+ compliant: Attributable, Legible, Contemporaneous, Original, Accurate—plus Complete, Consistent, Enduring, and Available. Personal data handling must align with GDPR requirements (where applicable).
7. Operational Readiness
Establish clear processes for handling inspections with readily accessible documentation. Your team should know exactly what to do when inspectors arrive.
8. Quality Assurance
Conduct regular internal audits, implement CAPA effectively, and maintain risk-based monitoring approaches consistent with ICH E6(R3) and WHO GCP guidelines.
9. Technology and Systems
Use validated systems with proper vendor oversight and documented user acceptance testing. Your technology should enhance, not hinder, compliance with 21 CFR Part 11 and EU GMP Annex 11 principles.
Practical Steps to Achieve Readiness
Transform these principles into action with these practical steps:
Create a Site Readiness Plan: Develop a comprehensive checklist covering all essential documents. Assign clear ownership for maintaining each aspect of inspection readiness.
Implement a Centralized System: Whether physical or electronic, maintain an organized filing system that enables rapid retrieval of any document an inspector might request.
Conduct Mock Inspections: Regular simulations identify gaps and build team confidence. Treat these seriously—they’re your best preparation for the real thing.
Establish an Inspection Response Team: Designate an inspection lead and create clear communication protocols. Everyone should know their role when inspection day arrives.
Embrace Continuous Improvement: Use inspection findings, whether from mock or real inspections as learning opportunities to strengthen your processes.
Critical Considerations for Success

Avoiding Two Critical Pitfalls
Pitfall #1: Inadequate Vendor Oversight Don’t assume your vendor is compliant—verify it. Request comprehensive 21 CFR Part 11, EU GMP Annex 11, and GDPR compliance documentation before implementation, not during an inspection.
Pitfall #2: Weak Internal Processes Having great technology means nothing without clear processes for using it. Develop comprehensive guidance for staff and maintain evidence that systems work as intended.
The Bottom Line
Inspection readiness isn’t a destination, it’s a journey of continuous improvement. By embracing electronic tools that meet 21 CFR Part 11 and EU GMP Annex 11 standards, maintaining comprehensive documentation, and fostering a culture of quality aligned with good clinical practice guidelines, you transform inspections from feared events into opportunities to showcase your excellence.
Remember, at its core, inspection readiness is about two fundamental principles: protecting study participants (21 CFR Part 50) and ensuring data integrity (21 CFR Part 11, GDPR). Everything else supports these critical goals.
The question remains: Are you ready for a clinical trial inspection tomorrow?
With proper preparation, the right technology, and commitment to these principles, you can confidently answer “yes” not just tomorrow, but every day.
At CRIO, we’ve built inspection readiness into the foundation of our platform. Our comprehensive document center, validation certificates, and built-in compliance features help sites, sponsors, and CROs maintain inspection readiness as a continuous state, not a last-minute scramble. Learn more about achieving inspection readiness with CRIO at clinicalresearch.io.
