A lot has been written about the eSource-to-EDC initiative, and while CRIO’s Central eSource initiative contributes significantly to this trend, the true value of eSource goes beyond merely capturing data to fill in the EDC.
There are 3 specific benefits sponsors get from Central eSource, above and beyond the acceleration of data entry and the need for reduced SDV from integration with the EDC.
1. Central eSource promotes protocol compliance
The EDC is a secondary entry tool, so none of the edit checks promote protocol compliance per se – they are merely tools to ensure accurate and complete data entry from already-collected source data.
eSource, by contrast, involves the contemporaneous capture of data while the procedure is occurring. Because of this, the edit checks in eSource do promote protocol compliance, as they provide real-time feedback to guide the site in the data collection process.
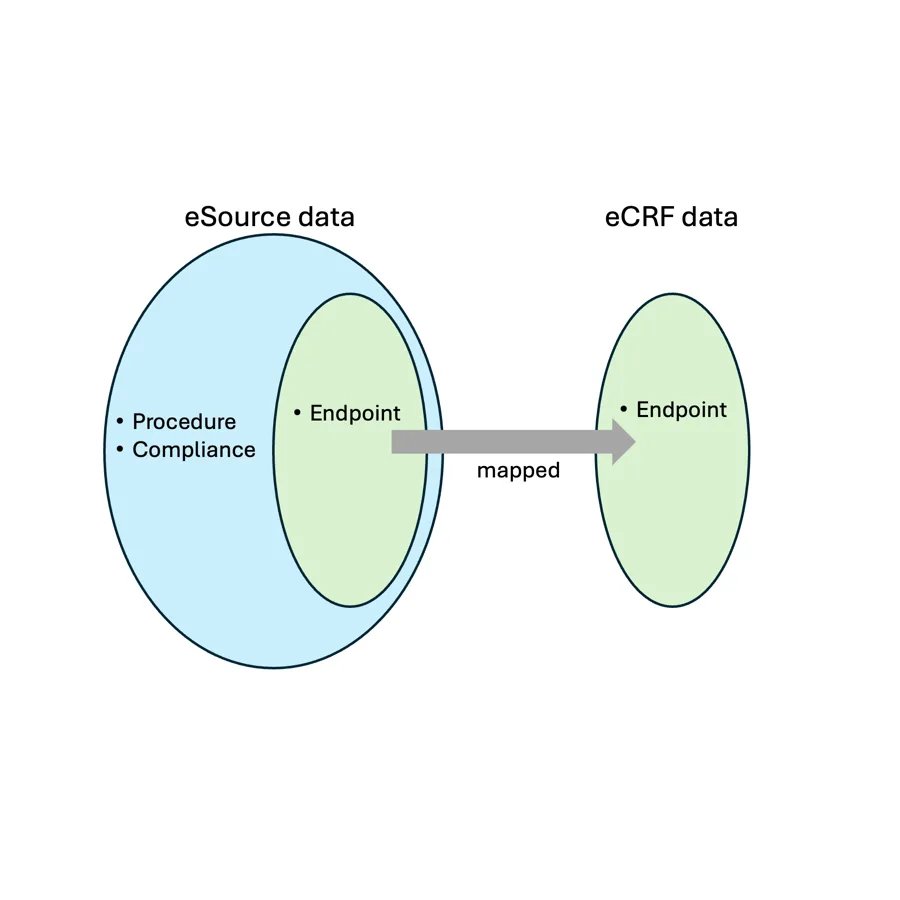
eSource data is broader than the EDC data in that eSource, when properly written, will contain the workflow instructions laid out in the protocol. By ensuring that the site is collecting the data in a standardized way, as directed by the protocol, eSource ensures that the subset of data that maps to the eCRF is in fact comparable to the data collected by other sites.
The following diagram visually depicts how eSource encompasses procedural and compliance data, not just the endpoint data frequently associated with the eCRF:
2. Central eSource facilitates site startup and enrollment
When a site receives the protocol from the sponsor after they are selected for a study, they need to translate it into a set of source worksheets. On paper, these worksheets could be 10 pages for a single visit; a study with 10 visits could easily result in 100+ pages of worksheets that need to be written.
Needless to say, this is an intensely time consuming process. CRIO’s experience, both with its own eSource design work and with talking to sites, is that a research coordinator may spend up to 35 hours writing complete source templates. That’s the billable time, not the elapsed time – needless to say, finding the time to carve out can be challenging.
As a result, a case study on Central eSource showed that sites who elect to utilize CRIO enrolled their first patient 41 days sooner than sites who elected not to – this represents roughly 7 weeks, which could easily be explained by the incremental lag it takes sites to “spin up” source.
Of course, sites could commence the source build before their Site Initiation Visit – but many choose not to, since they know from experience that studies can and do get delayed and withdrawn, and very few sites are willing to over-invest in study setup time prior to the Clinical Trial Agreement being signed.
Unless the study has a very short projected enrollment period (such as a flu vaccine study), sites have limited incentive to accelerate their timeline.
The same case study that showed sites enroll faster also showed that they enroll more – by 40% on average. This is not surprising, given that earlier enrollment should translate to greater enrollment overall, as it extends the recruiting window for sites and gives the site critical experience that compresses their learning curve.
3. Central eSource facilitates remote monitoring – faster EDC entry does not
Even with the advent of risk-based monitoring, site monitoring represents a significant portion of the overall budget of a study. Besides source data verification, monitors also perform source data review (“SDR”), which is the stand-alone review of a site’s source for consistent documentation, PI oversight, and compliance with the protocol and ICH-GCP.
SDR is a critical activity that unearths protocol deviations that may not be evident in the EDC. For instance, SDR may surface any of the following discoveries, all of which can undermine the study’s data integrity:
- Undisclosed medical conditions (eg, from a progress note, lab value, etc.) that indicates an improperly randomized patient;
- Lack of PI oversight, such as the lack of a PI involvement in critical documentation such as eligibility, medical history or adverse event review;
- Improper completion of a procedure, such as a source note that shows that endpoint data was not collected in the manner required by the protocol;
- Completion of a procedure by a staff member that was not properly delegated and/or trained (as source attribution is not apparent from the EDC).
SDR is a time consuming task and with a typical onsite monitoring cadence, many findings cannot be discovered until weeks, if not months, after the fact. For instance, one CRA reported that he learned about an undisclosed pregnancy – a major SAE/safety event – several months after the fact due to the lack of eSource for remote monitoring.
With Central eSource, the monitoring team has 24/7 access to the sites’ source, permitting a much faster, and more responsive, cadence of SDR. When combined with statistically driven RBQM tools, Central eSource empowers clinical teams to detect and prevent major deviations much earlier in the process, forestalling issues that could compromise the entire study.
Summary: Much more than populating the EDC
In another blog post, we demonstrated that Central eSource has the potential for higher data mapping with the EDC than EHR-based source systems. In addition to better EDC integration, the benefits described in this article are unique to Central eSource:
| EHR | Central eSource | |
| Protocol compliance | No impact. EHR systems cannot easily be configured for unique study requirements, so compliance is usually dependent on coordinator diligence adhering to paper-based “flowsheets” | High. eSource can be configured to induce protocol compliance, minimizing data quality issues. |
| Site startup and enrollment | No impact. EHR systems cannot inherit a centrally designed study template, so every site must develop their own protocol-specific flowsheets manually. | High. A centrally designed eSource can be built during the startup phase, then published to sites upon activation, eliminating weeks of delay. |
| Remote monitoring | Limited. Some EHR systems permit remote access, but many times, it’s time restricted and subject to complex site-specific access requirements. | High. CRIO’s Central eSource comes with a dedicated remote monitoring tool that makes site source available for review on a 24/7 basis. |
