CRIO Sites Out-enroll Non-CRIO Sites by 39%

Enrollment is critical to a trial, and site performance is a major – if not the most important – driver of that. We’ve always known that our full stack system enables sites to increase efficiency and enhance data quality, but do sites who use CRIO actually enroll more? We now have data to prove that CRIO sites do in fact have significantly higher enrollment rates than non-CRIO sites, in fact by almost 40%.
Measuring enrollment rate
To measure enrollment rates at CRIO sites compared to non-CRIO sites, we ran a report to find all protocols that have at least 10 sites within the CRIO U.S. database with enrolled patients. We then went to ClinicalTrials.gov to extract the estimated or actual total number of enrolled subjects and the total total number of sites in North America and globally
We excluded protocols that were actively enrolling because the CRIO enrollment statistics would not be comparable to the target enrollment rate on ClinicalTrials.gov. We also filtered out protocols that had less than 60% of sites within North America, to ensure geographic comparability.
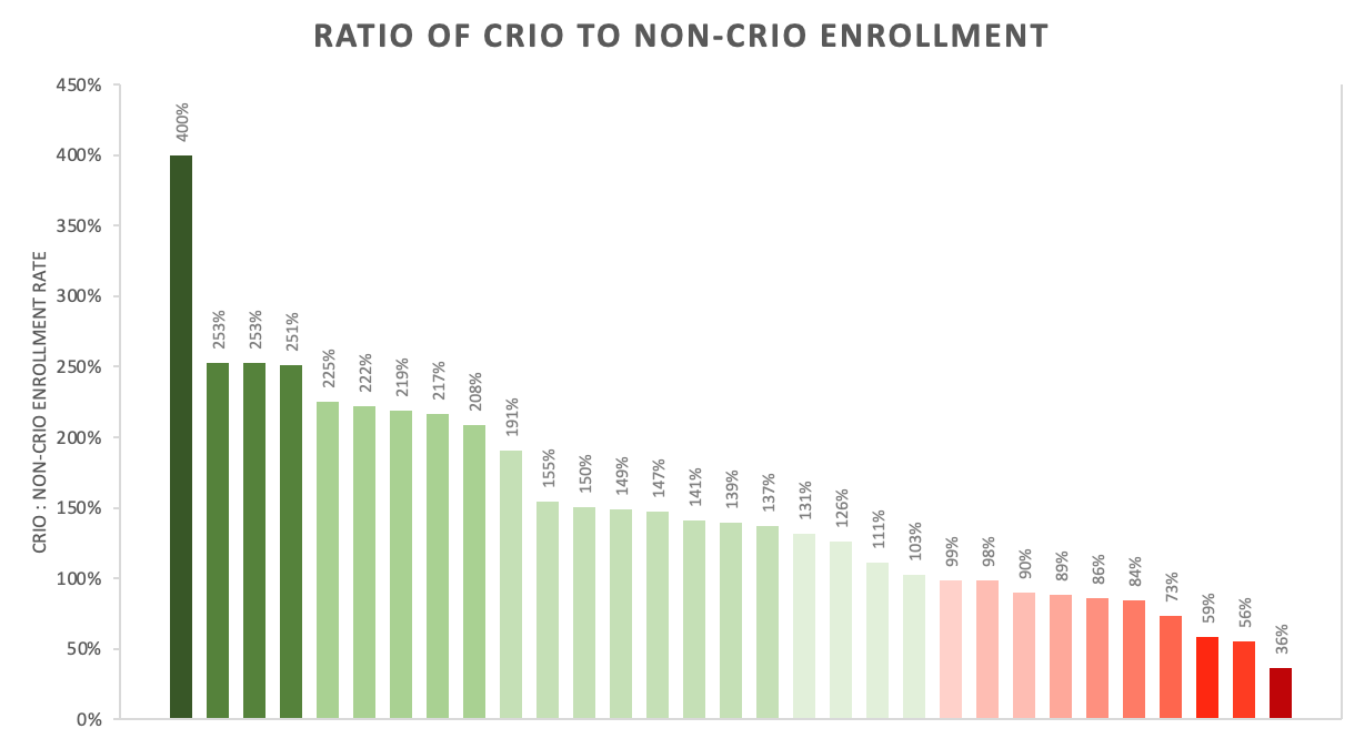
Ultimately, we ended up with 31 studies that fit all the criteria above. The enrollment rate of CRIO sites was calculated by dividing the total number of subjects randomized by those sites by the number of CRIO sites. The non-CRIO enrollment rate was calculated using the difference between the total enrollment and locations listed on ClinicalTrials.gov and those of CRIO sites.
The results
CRIO sites enroll 39% more than non-CRIO sites
We found that in 68% of the trials, CRIO sites out-enrolled non-CRIO sites, and the median ratio of CRIO to non-CRIO enrollment per site was 139%.1 Overall, CRIO sites made up an average of 22% of the total number of sites for each protocol, and accounted for 27% of all subjects enrolled. This means over 1 in 4 of total subjects went through the CRIO system. The protocols spanned 10 indications, with the leading indications in our 31-study set being gastroenterology and immunology.
Why do CRIO sites out-enroll non-CRIO sites?
We think there are two reasons CRIO sites out-enroll non-CRIO sites. The first is self-selection. By their very nature, sites who invest in technology are likely to be higher performing organizations with a strong desire to improve their operations. In fact, CRIO has a very high share – over 50% – among the emerging class of site networks that are bringing standardization and professionalization to the industry.
Second, CRIO’s full-stack software enables efficiencies, freeing up site staff from clerical and administrative tasks so they can redirect their time and energy to patient recruitment and retention. With finance, scheduling, and eRegulatory workflows fully integrated into the eSource, processes like re-consenting patients, calculating visit windows, and logging receivables for billing become automated for site staff. CRIO’s seamless recruitment module, which includes a one-of-a-kind API, enables more efficient recruiting.
CRIO’s influence is growing rapidly in the industry as more sites and site networks implement CRIO into their operations. We’ve always known that our innovative solutions attract sites run by forward-thinking, ambitious people who look to be top-performing sites. In turn, our system enables them to outperform and out-enroll other sites by increasing their efficiency. Sponsors should recognize that CRIO sites are higher performers – simply by selecting more CRIO sites on their trials, they can shorten their enrollment timelines!
- Due to the wide range of study sizes, we used the ratio of medians to eliminate the outliers that could disproportionately impact a mean approach.




