CRIO’s integrated eSource-EDC Model is a Game Changer

CRIO’s integrated eSource-EDC model revolutionizes both the data entry process at the site level and the monitoring process from the CRO perspective. According to a third party survey of CRAs who have used CRIO, CRAs agreed by a margin of 23:1 that this new model would increase overall trial efficiency, improve data quality, and enhance site performance.
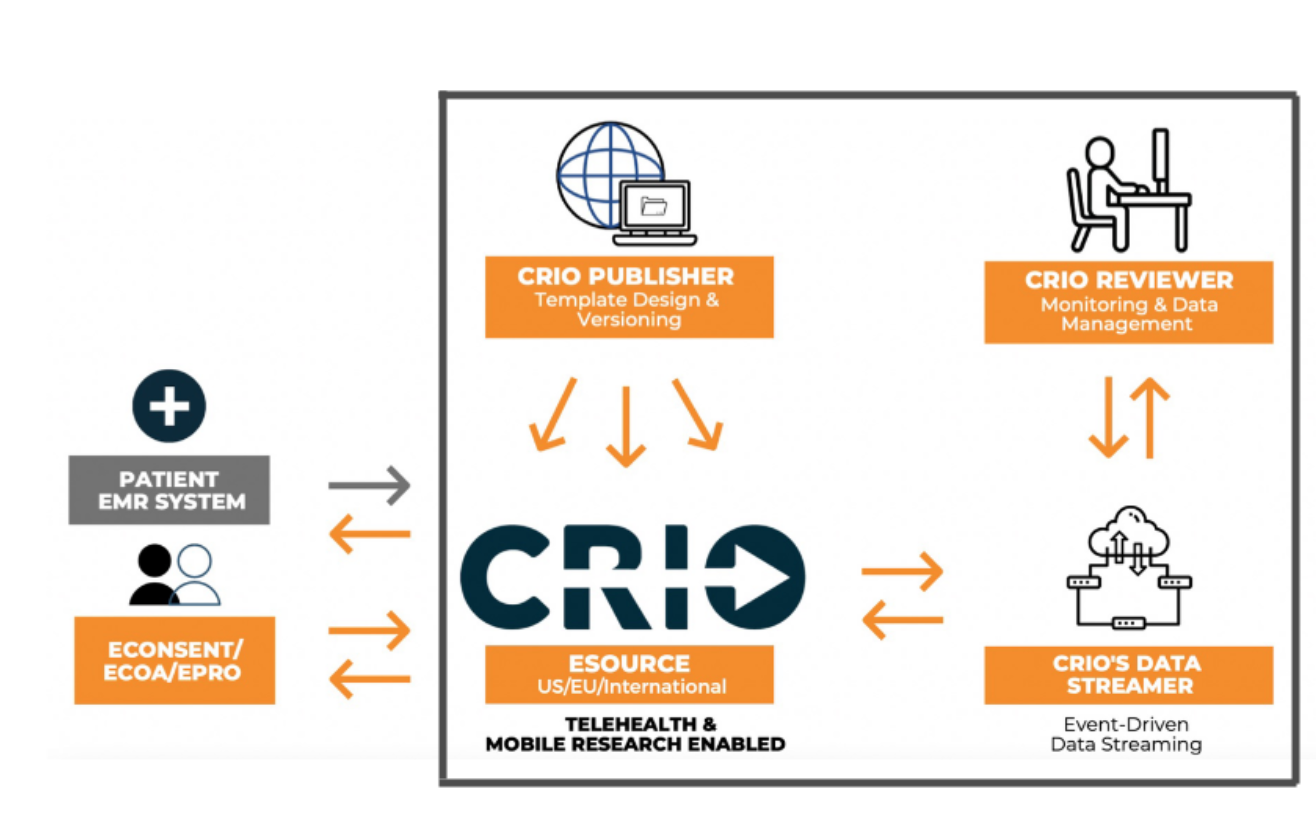
The integrated eSource-EDC system
In CRIO’s integrated eSource-EDC system, source data that is collected by the site gets transmitted and anonymized through CRIO’s data streamer and becomes available for monitors and data managers in CRIO’s Reviewer EDC module. This reimagines the data entry process by completely eliminating the transcription process from source to EDC as the eSource effectively becomes the EDC data.
About the survey
CRIO retained a third party market research firm to conduct a web-based survey of Clinical Research Associate (CRA) users with recent logins to the CRIO system. The firm solicited participation from 03/16/2022 to 03/30/2022 to an anonymous web survey. The survey received a total of 79 responses. All users were assured of anonymity. CRIO did not have access to the identifying information of any respondents.
The survey reflected favorable perceptions of CRIO from its CRA users. At the time of the survey, we proposed the eSource-EDC integrated model as CRIO’s Reviewer EDC was preparing for its official release. We asked if CRAs anticipated an increase or decrease in various study performance metrics after implementation of CRIO’s integrated system.
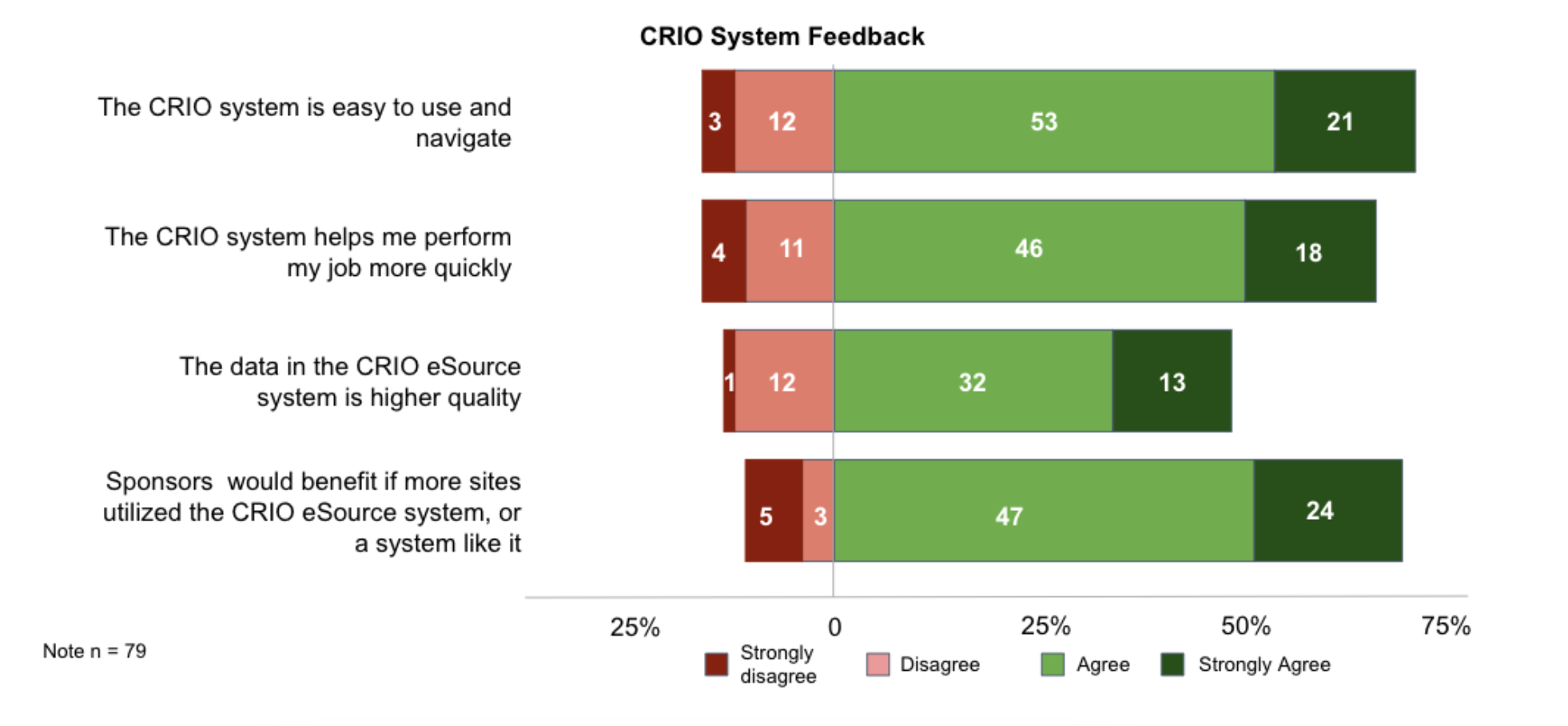
CRA’s agreed that the new integrated eSource-EDC system would increase all of the proposed metrics:
Improve overall data quality and reliability (23:1)
By an overwhelming margin of 23:1, more CRAs anticipate an improvement in overall data quality.
CRIO’s eSource has always provided automated edit checks to enforce protocol compliance and ensure data quality. Sites can configure every question with instructions and built-in alerts that will immediately catch entry errors, protocol deviations, and possible adverse events indicated by out-of-range values.
But CRIO’s new integration of eSource with EDC now enables an additional level of quality-assurance. Using Reviewer EDC, CRAs can view the visit data immediately, and then review, issue queries, track source changes, and lock source. This means that the CRO can perform contemporaneous monitoring, thus catching protocol deviations, subject exclusions, and adverse events sooner, preventing further mistakes down the road and protecting patient safety.
Accelerate data lock (18:1)
CRAs agree by a margin of 18:1 that the CRIO system could accelerate data lock. This is because in addition to facilitating contemporaneous review by the CRA, the system also secures PI sign-off on the source. This leverages the PIs’ existing practice of signing off in source to evidence PI oversight. When the PI has signed off and staff has resolved all queries, the system will allow the CRA to lock the visit. This means that locking is now a continuous event, thus facilitating much faster database locking.
Enhance site performance (11:1 and 9:1)
CRA’s anticipate the integrated model to enhance site performance both from freeing up time for CRAs (by a 11:1 margin) to work with sites to improve performance, and from giving time back to site staff themselves (by a 9:1 margin). With cleaner data, smaller workload without SDV, and no travel time, CRAs can re-allocate time to train and engage with site staff. This promotes more protocol compliance by sites and faster turn-around on key questions from sites. For sites, eliminating EDC entry frees up time for them to carry out other study duties like recruitment, query management, and conducting study visits.
Increase overall clinical trial efficiency (5:1)
By a 5:1 margin, CRAs anticipate an increase in the overall clinical trial efficiency with the integrated system. The increased efficiency is due to reduced monitoring travel time, reduced time spent on data management, and the elimination of source data verification.
The traditional model of housing two of the same data set separately in the EDC and eSource creates redundant processes for both the site and CROs. CRIO’s integrated eSource-EDC model is a game changer as it unlocks significant time savings for both parties and ultimately produces higher quality data for the sponsor.




