The eConsent software that integrates with your eSource platform to automate your informed consent form (ICF) management is clearly CRIO.

ICF Management Made Easy
Informed consent is one of the most critical components of any clinical trial —and also one of the easiest places for protocol deviations to occur. Electronic consent (eConsent) forms reduce patient and site error. But missed re-consents can still happen unless your platform is built to prevent them.
Eliminate missed re-consents with CRIO’s integrated eSource and eConsent software.
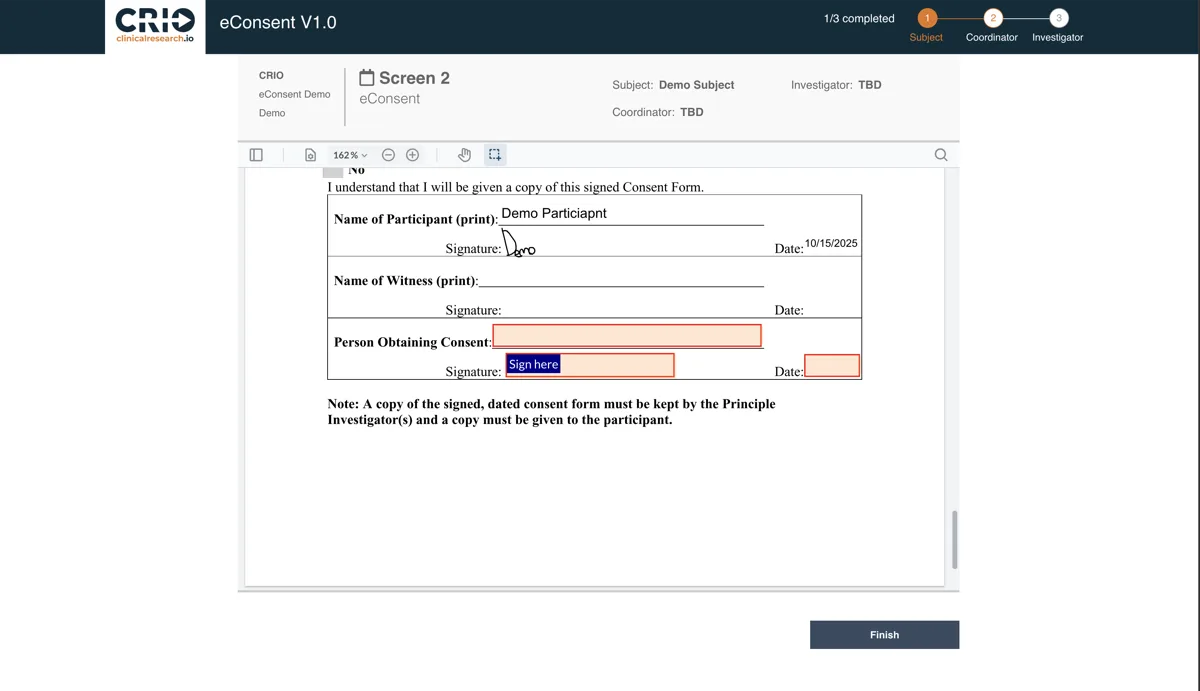
Because CRIO eConsent is embedded directly into your eSource workflow, there is no need for toggling between platforms or manually checking ICF status. Every visit, every patient, and every consent is automatically accounted for — while ICH, FDA, and IRB expectations are met:
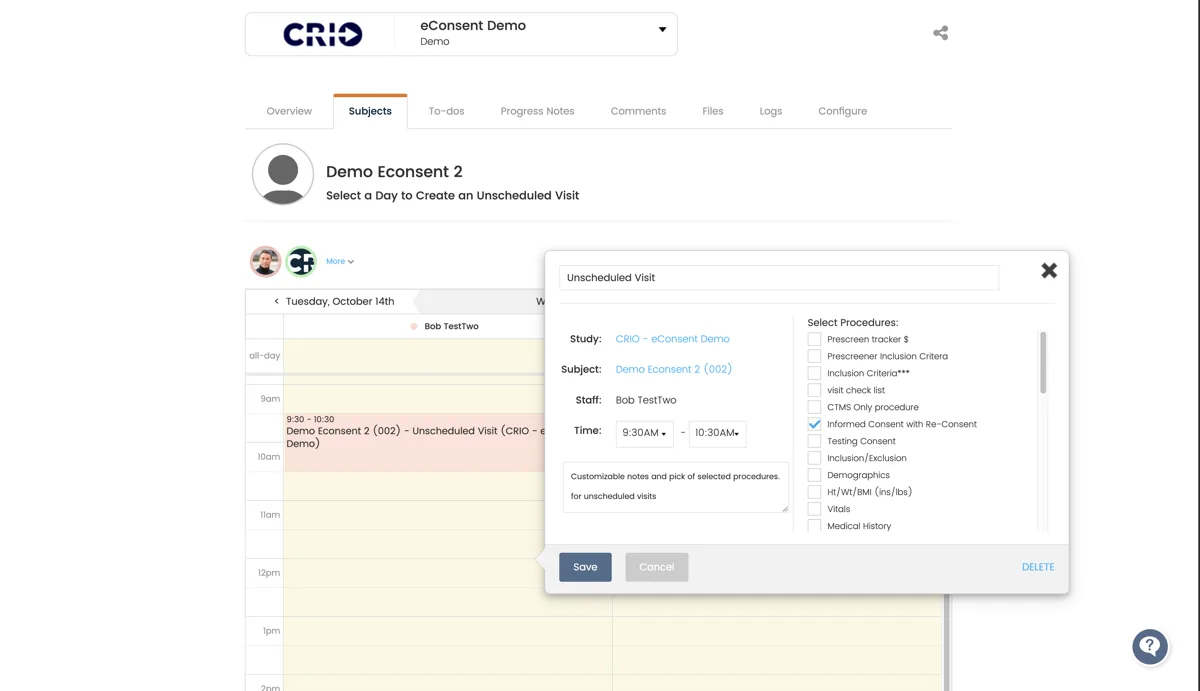
- Configure, execute, and track eConsent forms
- Reduce the risk of missed re-consent deviations
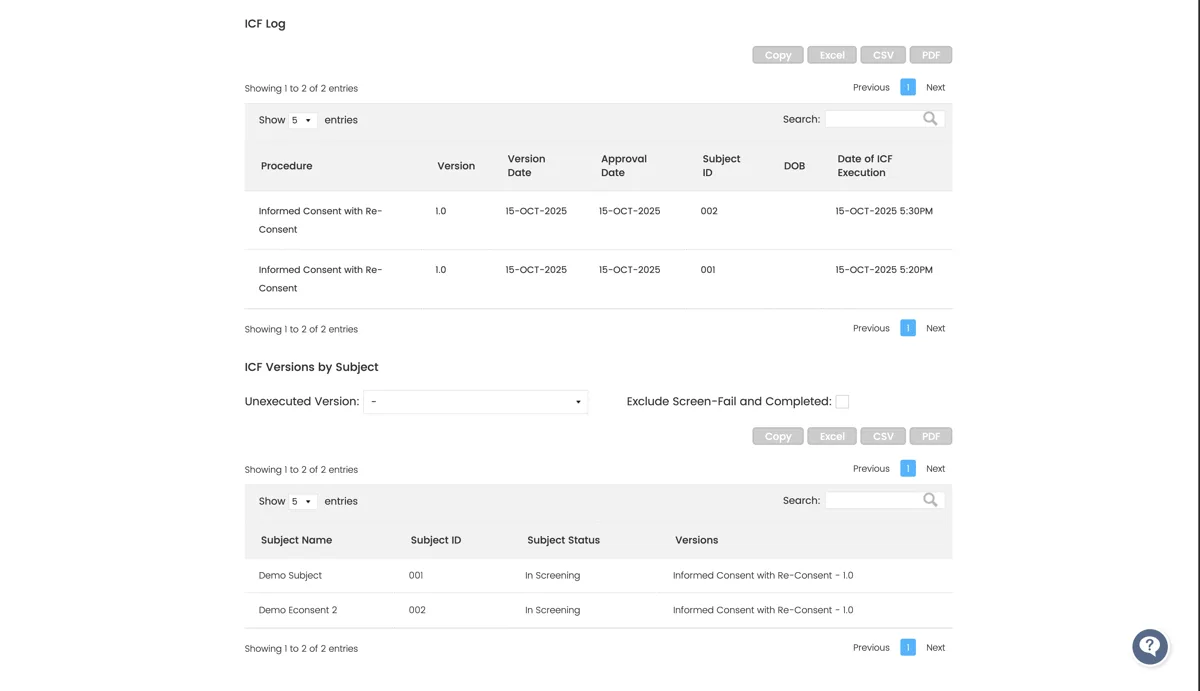
- Automatically track which patients have executed which versions
- Optimize ICF management without lifting a finger
How It Works
At the outset of each patient visit, CRIO automatically checks if the patient has executed the most recent ICF.
If the patient has not consented under the current ICF, CRIO will notify the clinical research coordinator and provide a link to open the associated eConsent or initiate a remote consent procedure.
Complete eConsents automatically flow into Reviewer EDC for remote monitoring, giving CRAs a single place to view subject source and executed forms.
It’s that simple.
What You Can Do With CRIO’s Integrated eSource-eConsent Software
With CRIO, ICF management means:
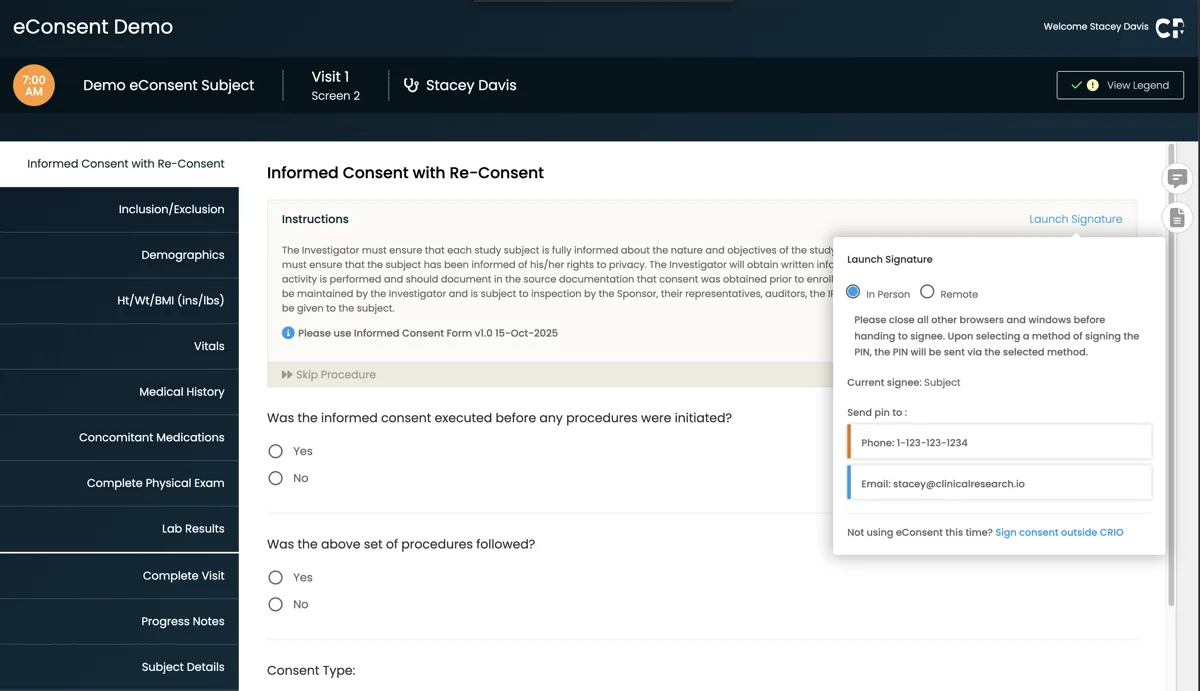
Remote and On-the-Go Consenting
- Patients can review and sign consent forms from anywhere — no in-office visit required
- Consent forms render seamlessly across devices, including smartphones
- Patients can complete consent processes outside of standard clinic hours
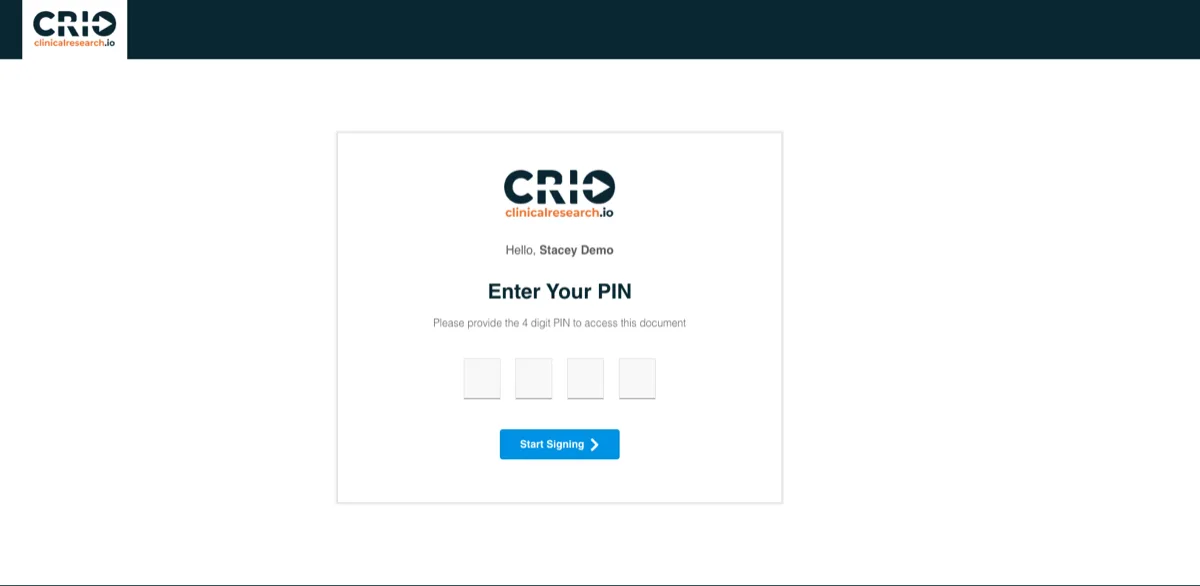
Secure Patient Verification
System-generated PIN codes ensure patient identity during remote consent.
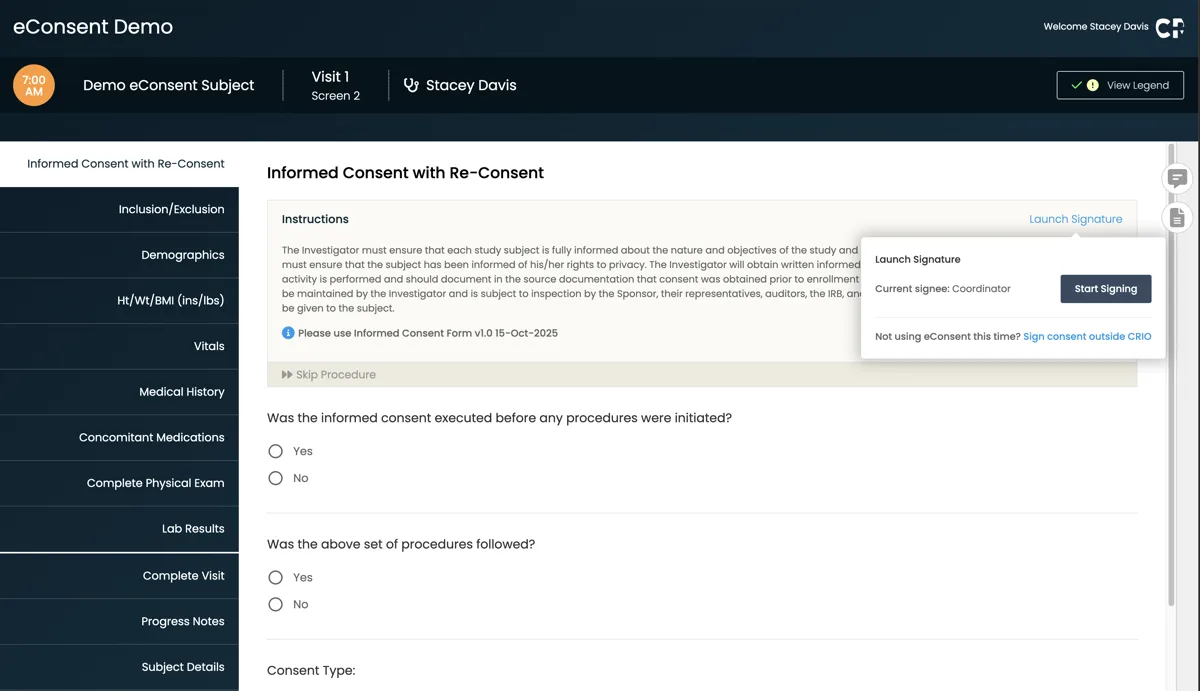
Streamlined Workflow
Coordinators can send, review, and countersign consent forms electronically without managing separate platforms.
Faster, More Flexible Scheduling
Eliminates 1.5-hour pre-consent appointments.
Version Control
Ensures that patients always sign the latest IRB-approved form, no manual tracking needed.
Improve Compliance and Patient Experience
Supports protocol adherence while making participation easier and more convenient for patients.
How Automated ICF Management Facilitated a 200% Increase in Patient Enrollment
When traditional workflows slowed enrollment and strained resources, Innovo Research turned to CRIO’s integrated eConsent solution and saw immediate results. From reducing patient burden to enabling after-hours consent, this case study reveals how one site tripled enrollment in a high-impact cancer screening study.
Download the case study to see why CRIO’s eConsent tool is an integral part of modern clinical trials.