Your 21 CFR Part 11-Compliant eSource Platform
It’s easy to assume that clinical research data live in electronic health records (EHR) and just need to be mapped to your electronic data capture (EDC) system. But research data often end up in paper documents because EHR systems are designed to capture records related to diagnoses and patient care, not research.
There’s proven clinical research software that can close this gap and alleviate the burdens of conducting research within your institution. It’s clearly CRIO.
-
40% fewer protocol deviations
-
70% lower audit risk
Dig deeper into the differences between EHR and eSource and how CRIO bridges the gaps.
Intuitive Clinical Research Software Enabling Efficient Protocol Compliance
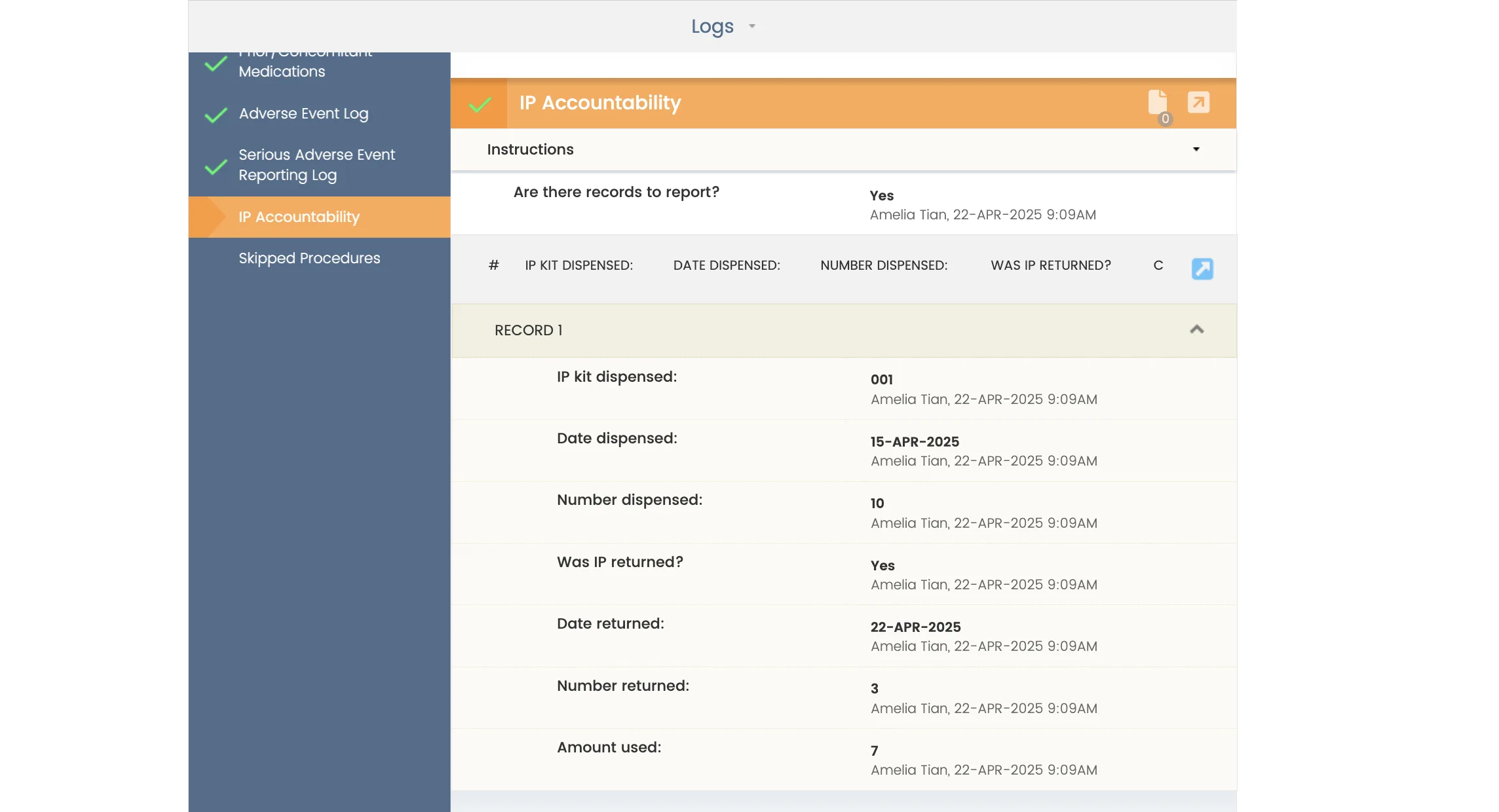
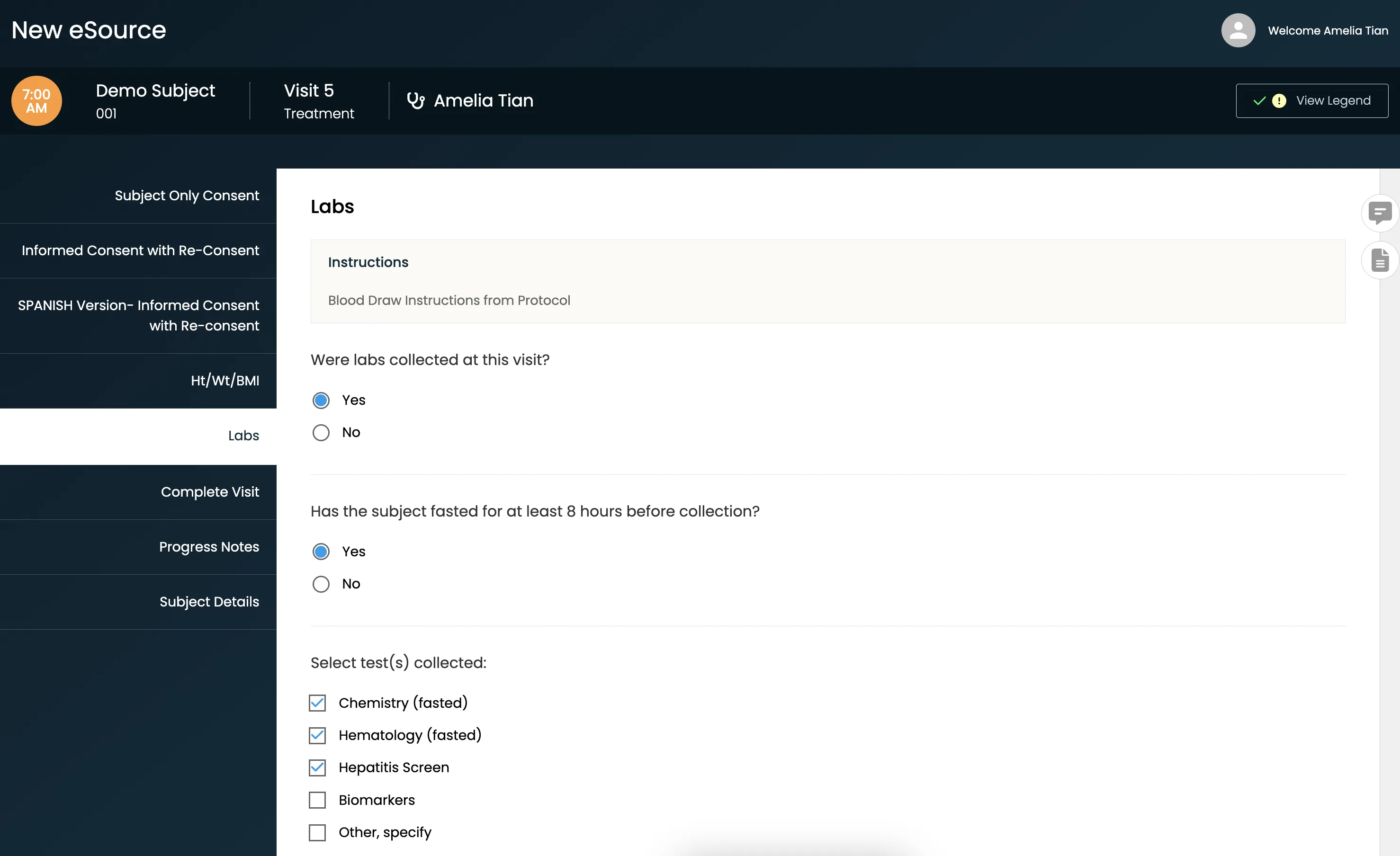
A powerfully simple eSource technology created by a team of clinical research veterans, CRIO helps you ditch paper, make the most of the systems you already have, and empower your teams.
Collaborate Remotely
- Close the gaps between EHR systems, paper source documents, and EDC
- Streamline principal investigator (PI) review of lab data and medical records
- Provide convenient remote monitoring for clinical research assistants (CRAs) while reducing coordinator burden
Easily Run Investigator-Initiated Trials With Integrated EDC
- Quickly configure and deploy eSource templates without any programming requirements
- Auto-populate EDC with PHI-protected data as you complete source templates, eliminating transcription and source data verification (SDV)
- Support single and multisite trials, including those requiring 21 CFR Part 11 compliance
Safeguard Security & Quality
- Meet compliance guidelines, including 21 CFR Part 11

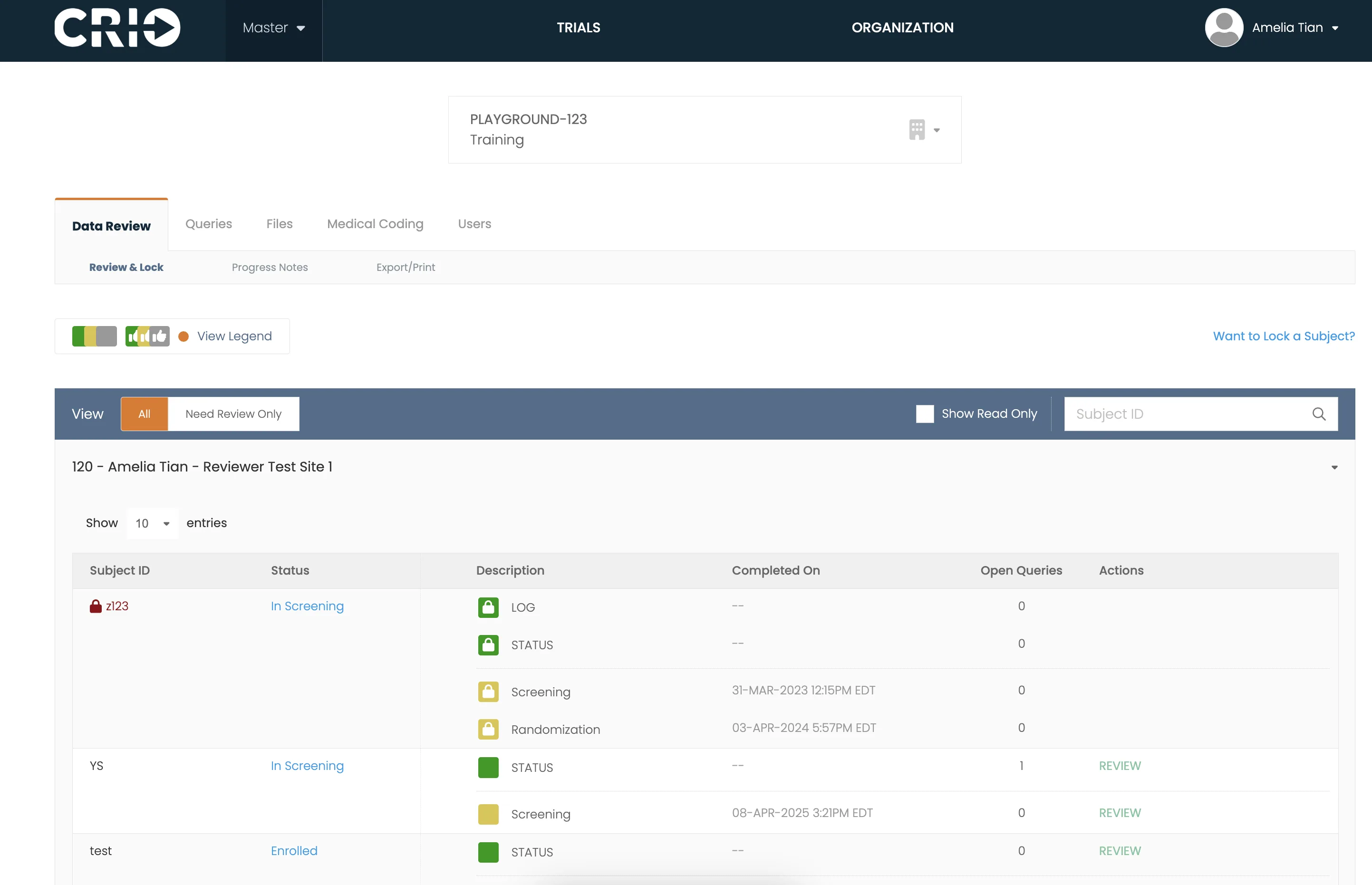
Case Study
This University Saves 650+ Hours of Coordinator Time Every Year
Get an inside look at what happens when academic research centers go paperless with CRIO eSource software.