How Monitors Spend Their Time, And How That Can Be Cut In Half

Note: As of this writing, CRIO’s Reviewer EDCmodel (eSource/EDC) is live in several studies; early indications are that the new operating model saves sponsors and CROs significant monitoring time. In this post, we outline the current CRA time allocation as a baseline, then model how our innovative approach can cut that time in half.
Clinical Research Associates (CRAs) play a vital role in maintaining and verifying the quality of collected data. In the traditional EDC model, a monitor’s duties include traveling to various cities to verify all aspects of the study protocol with site source documentation. This includes performing source data review (SDR), confirming protocol adherence and patient eligibility, and source data verification (SDV) against EDC.
However, this traditional model has been historically time-consuming and inefficient. Not only does a monitor have to be on-site to perform these extensive verifications, but the inconsistent time intervals between data collection, EDC entry, and monitoring visits can leave data discrepancies undetected for months.
Our CRA time survey
CRIO eSource has been deployed on thousands of studies – well over a thousand CRAs have used CRIO eSource to perform monitoring. Against this database of CRA users, we sponsored a web-based survey through a third-party market research firm to gauge CRA feedback on the system and gather a baseline on how CRAs spend their time. The survey was anonymous and solicited responses from 03/16/2022 to 03/30/2022.
As described in other blog posts, the survey results were overwhelmingly positive. They demonstrated that CRAs perceived CRIO as easy to use, improved data quality, and most importantly, increased the efficiency of their workload.
Notably, the survey also gave CRIO a baseline of how CRAs allocate their time. CRIO uses this baseline to model how that time distribution might change by using the continuous remote monitoring process at the heart of our CRIO eSource-EDC model.
Traditional Model vs. CRIO eSource-EDC Model
In the traditional model, CRAs perform SDR and SDV, and Data Management (DM) performs a form-by-form, field-by-field review of the data entered into the EDC. This DM review function relies on manual edit checks for the accuracy and consistency of data. However, most of these DM edit checks are already part of the monitoring plan adopted by Clinical Operations, only against the underlying source data. So when the source and the EDC data become one, the DM review function can now fold into the CRA SDR function. We call this new role “Clinical Data Monitoring” to indicate that the review is to include both the clinical elements (e.g., eligibility, safety, protocol compliance) and the data integrity elements (accuracy and consistency).
Using conservative assumptions, we predict that over time, the Clinical Data Monitoring role would eliminate half of CRA time through savings in three main areas: SDV, travel, and administrative duties. This represents a significant amount of hard cost savings for sponsors, as monitoring is often the largest line item on a CRO’s budget, and the labor and travel alone could constitute up to 30% of the cost of the entire trial.
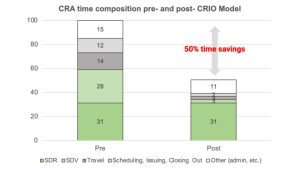
In the following analyses, CRIO will quantify time spent on activities relative to an index of 100. Thus, since SDV currently constitutes 31% of CRA time, we describe total CRA time as 31 units, making our metrics easier to follow.
Source Data Verification (SDV) and Source Data Review (SDR)
The survey revealed that CRAs spend 31% of their time on SDR and 28% on SDV. However, with CRIO’s eSource model, most SDV is eliminated from the process since eSource becomes EDC data, except for a few clinical data points that might be captured outside of CRIO, then uploaded as a PDF. At the same time, the coordinator transcribes the data into the eSource/EDC. Even with SDR, the process becomes faster, data is cleaner, and the presentation is standardized.
By adopting CRIO’s new integrated eSource-EDC model, we project the elimination of 90% of SDV, meaning that SDV time would decline from 28 to 3, a savings of 25. We can also project a reduction in time spent performing SDR because this model standardizes the templates and the data is inherently cleaner – but, to be conservative, we won’t. Instead, we’ll presume that the Clinical Data Monitoring resource will be more thorough in interrogating the source data to confirm protocol and ICH-GCP compliance, subject eligibility, and patient safety – time that is well worth the investment.
We recognize that CRAs do become backlogged and may need to take shortcuts to perform their core SDV duties. By eliminating the distractions of SDV and the inherent inefficiencies of onsite monitoring (described next), our model allows the monitoring resource to focus as much time as needed on the critical elements of the trial.
Travel
In the traditional model, CRAs are expected to travel to sites for monitoring visits. These visits can be time-consuming and costly, particularly when involving multiple sites. According to the survey, CRAs spend 14% of their time on travel. With CRIO’s Reviewer EDC tool, most monitoring visits become unnecessary as monitors can execute contemporaneous data review. Instead, CRAs can utilize onsite visits more strategically, such as for site training/initiation, Investigational Product (IP) accountability, or as part of a risk-based monitoring (RBM) approach. In the CRIO model, we eliminate 80% of onsite visits via our assumptions, thus reducing travel time from 14 to 3. Not only is this a significant time saving, but it also saves sponsors substantial travel costs passed through to them.
The Hidden Costs of On-Site Monitoring
There is one more efficiency related to travel that our model cannot fully capture, but it is real. In the traditional on-site visit, the CRA only has 8 working hours (9 to 5) to perform his or her responsibilities. In the real world, it’s often challenging to predict exactly how much time is required to do monitoring, especially while enrollment is ongoing and the CRAs have not been able to get a baseline on individual sites’ performance. This means that the CRA may not have enough time to complete their duties, thus requiring a second day or return trip. Or, worse yet, the CRA defers the needed review of the data until the next on-site monitoring visit – making that review even more stale, and potentially delaying protocol deviation detection even further out.
On the flip side, we know of many anecdotes where the CRA finishes early. However, we suspect some CRAs will still log a full 8 hours in billing time since there is often no automated time clock that tracks their actual working time on-site (or, for that matter, if they’re even doing the study sponsor’s work). The current monitoring model provides limited accountability, where the source data is onsite, and the CRA often travels solo.
Administrative Duties
In the traditional model, administrative duties such as scheduling, issuing follow-up letters to sites, and closing out action items account for 12% of the total time spent on tasks. Follow up letters document what visits the CRA reviewed and their findings. The CRIO model embeds the CRA’s review and query activity within the Reviewer EDC application itself, so the only documentation that the CRA would need to provide relates to conversations with site staff or findings pertaining to non-source data. Thus, with the reduction of travel and elimination of most follow-up letters and other checklists, we estimate that the time spent on these activities could decrease by at least 50%, or from 12 to 6.
Additionally, CRAs spend approximately 15% of their time on other miscellaneous administrative tasks not included in the survey’s specified categories. Since this overhead time is often associated with the underlying time requirements for core activities, we can conservatively model a 25% reduction in this category, or from 15 to 11.
Conclusion: We project 50% CRA savings from our model
All told, we project 50% time savings across the activities mentioned above. This estimate assumes no reduction in time spent on SDR, although, with cleaner data, more standardization, fewer deviations, and the ability for CRAs to prevent repeat deviations, we foresee that SDR, itself, could go down in time. Our model significantly reduces the overall cost of conducting a study by eliminating half of monitor billing time, 80% of travel cost, and a substantial portion of the DM billing time.
With aggregate cost savings of up to 20%, our model can increase the R&D pipeline for a sponsor by a factor of 25% – in other words, a sponsor that would typically budget for 8 trials a year could now budget for 10 a year at the same spend level. This also does not account for the evidence that sites enroll more when liberated of time-consuming clerical tasks – so with higher enrollment velocity, trial costs come down even more. This R&D throughput increase has vast implications for an industry struggling with ever-rising costs and stagnating productivity.
Ready to learn more? Schedule a demo to explore CRIO.




