Active Trials Per Site Are Still Decreasing From COVID-19, Survey Finds

At the end of March 2020, Clinical SCORE conducted an online survey to gain insight on how the pandemic is affecting clinical research sites across the world. As the crisis is still evolving, the company conducted a similar questionnaire at the beginning of May to obtain a more updated picture of clinical trial sites and to compare impact at the two time points.
In terms of the number of active studies that each site has, the data shows that sites are now managing significantly fewer active trials.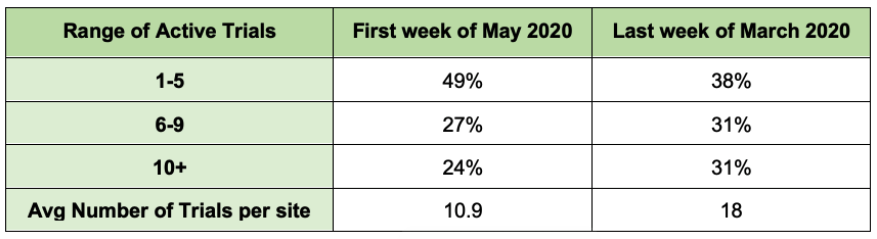
For active studies that are enrolling and randomizing patients, there was no significant difference in impact between late March and early May. Sites listed the following reasons for substantial delays to these studies:
- Patients are unable to come to site (the number of sites that reported this increased from 48% to 64%)
- Protocol amendments by sponsors (the number of sites that reported this increased from 11% to 22%)
For studies that are not yet enrolling participants, 51% sites indicated that delays are due to sponsors postponing these trials. This represented a 15% increase when compared to the end of March.
The survey also revealed that the number of protocol deviations has decreased over the past month. Protocol deviations are most notably due to implementation of home visits in place of on site visits.
In regards to impact on trial staff, a third of participating sites indicated that the impact has been extreme. While staying on top of changing protocols and procedures remains the number one reason for staff impact, Clinical SCORE observed a significant increase in percent of sites concerned by economic realities (26% to 41%).
To gauge levels of patient nervousness about returning to site for future study visits during the ongoing pandemic, Clinical SCORE added a new question that asked sites to rate on a scale of 1 to 7, how they believe study subjects would feel about on-site visits. They found that free-standing research sites were much more optimistic about subjects coming back to site. Unfortunately, the results did not break down the datapoint by therapeutic focus or by geographic location. Future surveys should investigate whether patient sentiment depends on site location (urban vs. rural) and/or by medical specialty.
As clinical research sites continue to navigate this unprecedented situation, they hope that sponsors and CROs will…
- Provide additional guidance on trial maintenance (e.g., how to utilize alternative methods for site visits, how to comply with the time constraints of protocols)
- Offer more guidance on how to conduct virtual, telephone or home visits while following study protocols
- Provide financial assistance
- Exert better control over study supplies
- Provide information on safety guidelines for staff and patients
- Be open to protocol changes that sites identify as necessary
This survey was fielded during the first week of May 2020 with 226 clinical trial sites across 38 countries. 119 of the respondents participated in the first survey.
For more information on the participating sites and to see the full results of the survey, visit Clinical SCORE’s website.




