eSource: The Platform of the Future
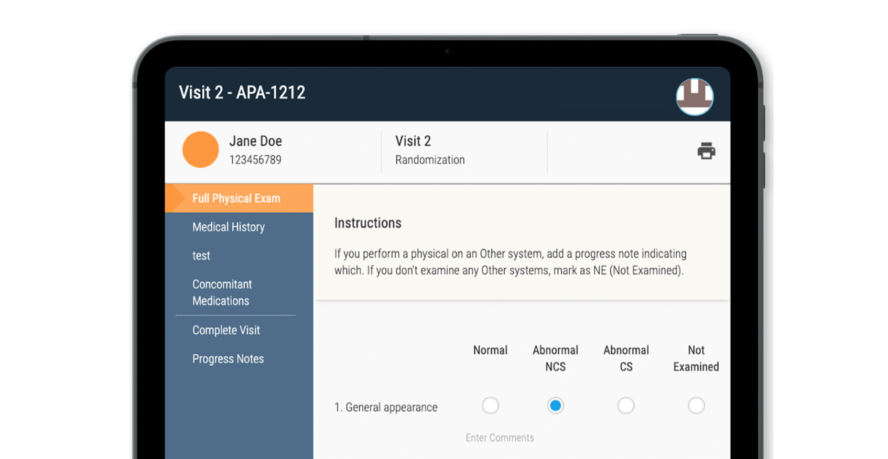
In the latest virtual conference hosted by ACRP, Innovation in the Era of COVID, Raymond Nomizu of Clinical Research IO and Jessica Branning of ClinCloud Clinical Research discussed electronic Source (eSource) and how it has shaped the future of clinical research.
Ms. Branning’s site, ClinCloud, was built ground-up on a technological platform centered around CRIO. Her site, being fully electronic, was well prepared for the changes the pandemic brought to the industry, and she provided a case study to validate many of Mr. Nomizu’s points.
According to Mr. Nomizu, the first real evolution of clinical research technology occurred with the development of clinical trial management software (CTMS), or what he likes to call ‘back office’ technology. Previously relying on external programs for planning and management of their clinical trials, research sites were finally equipped with software capable of various features like financial management, scheduling, and patient recruitment.
We are now in the second phase of technology evolution with the development of ‘exam room’ technologies such as electronic source (eSource), electronic regulatory systems (eRegulatory), and electronic consent (eConsent). The number of sites that have adopted an eSource platform has increased exponentially.
What are the reasons behind this trend?
1. Increased Efficiency
With the elimination of paper-based processes, research sites are able to save time.
2. Increased Quality
Workflows are automated and the number of deviations is reduced.
3. Increased PI Oversight
All principal investigators and qualified site personnel have 24-hour access to data and resources.
Looking at broader trends in the industry, each trend also points to the use of eSource.
Virtual Monitoring
Throughout the COVID-19 pandemic, pharmaceutical companies and CROs turned to virtual monitoring. In doing so, clinical research associates or monitors were able to perform a tremendous amount of monitoring without the physical travel necessitated by an on-site visit. As eSource is a method of direct data capture, it allows instantaneous and continuous access to subject data and is superior to scanning and uploading paper source charts. With all data electronically captured, the issue of legibility is completely eliminated and data verification processes for monitors are streamlined.
Ms. Branning explained that with CRIO’s eSource system, the amount of time needed by monitors to review data during a remote visit was 2-3 hours shorter. “Monitors also like having access to our eSource system,” she said, “as they can ping coordinators directly in the internal communication system and efficiently resolve queries.”
Rise of Site Networks
With the formation of site networks, research sites are able to identify and standardize best practices using technology. Before the creation of ‘exam room’ technology, each site had to house a large number of local staff to get things done. In aggregating resources across multiple locations in a site network, eSource and eRegulatory allow for the centralization of functions and resources, including not just business development and budgeting, but also regulatory compliance, source template design, quality control, EDC entry, and financial management. “In other words, eSource allows for smaller, but more powerful footprints in multiple locations,” says Mr. Nomizu. Ms. Branning points out that she is able to operationalize research at new physician practices much more quickly as a result.
Integration
Integration refers to the ability of different systems (e.g., eSource, CTMS, EDC, IRT) to talk to each other. As site workflows are at the heart of all clinical research, it makes sense that site systems should integrate with others. The flow of information between patients, sites, and sponsors will allow for greater efficiency and accuracy. Although the industry is only in the beginning stages of exploring integration, the end result will be a fully stacked solution available to clinical research sites worldwide. As Ms. Branning says, “Everyone should welcome this with open arms. Yes, there will be challenges, but once integrations are established, it will be golden.”
Panel Members
– Raymond Nomizu, Co-Founder and CEO of Clinical Research IO
– Jessica Branning, CEO of ClinCloud Clinical Research
View the entire ACRP webinar here. The replay is free however you must create an ACRP account to gain access.




