Progress Notes are Vital to Source Data

Progress notes are free-text entries by the investigator, coordinator or study team member that are inserted into the source record. Generally, these play a critical and highly undervalued role in the study process. Progress notes are often used to:
- Clarify or confirm any data points that may appear as outliers, even before a query is issued.
- Explain the clinical reasoning behind a PI’s assessment of eligibility, safety or patient medical history. This includes discrepancies or gaps in the EMR.
- Document the steps a site has taken to mitigate a protocol deviation or safety event.
Overall, progress notes serve as vital evidence of effective PI oversight.”I teach all our CRA students about the importance of progress notes as a documentation tool for PI oversight,” said Dan Sfera, head of The CRA Academy, a training program for new CRAs. “A well-written progress note can make the difference between an unexpected, but minor, data discrepancy and a major protocol deviation that requires sponsor escalation. Without progress notes, monitors would not be able to fully confirm critical elements of the protocol, such as eligibility, protocol compliance or patient safety.”
EXAMPLE: In the example below, the PI has consolidated two items from the patient’s electronic medical record into one medical history item. Here, the PI documented his/her reasoning in a progress note. Without the note, an auditor could flag the discrepancy as a protocol deviation, casting doubt on the integrity of the source data.]
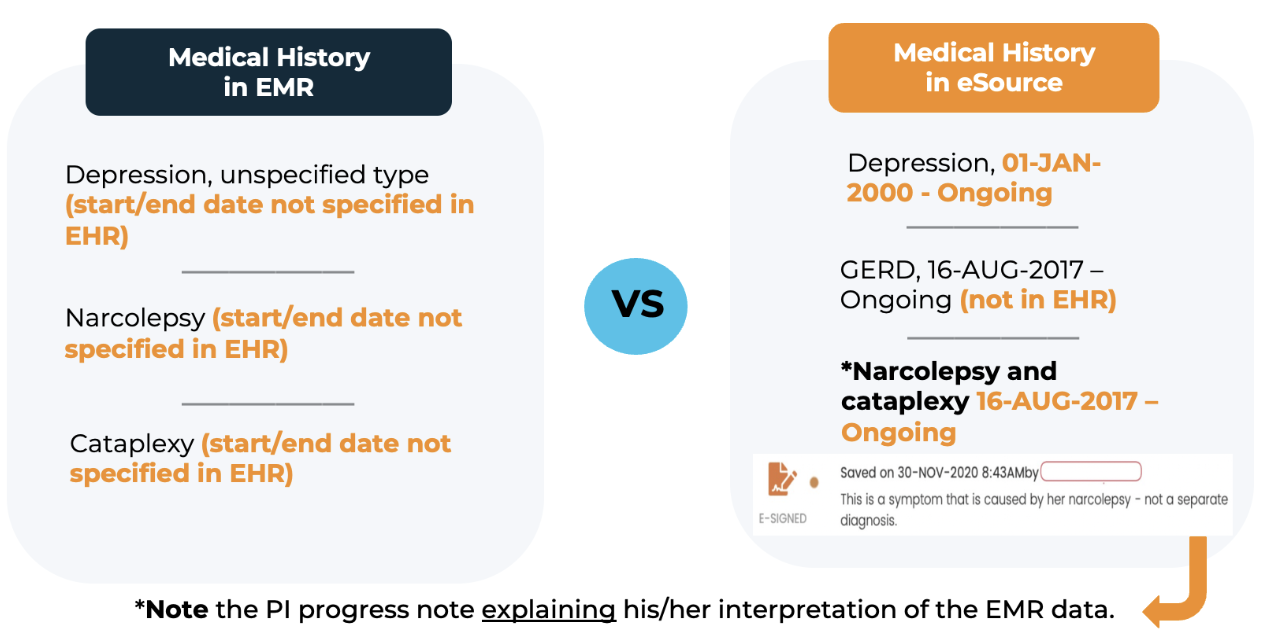
Because progress notes can be applied to any data point in real-time, CRIO eSource provides a far richer source of study data than the eCRF.
Benefits of eSource vs traditional eCRF
CRIO’s eSource system lets sites capture source data in real-time. Edit checks are applied at point of data capture to ensure protocol compliance. However, sometimes additional information is required to explain an event or data of a study participant. At any time, sites are free to add progress notes to a specific procedure within a visit; for a specific visit; or for a subject. CRIO client sites use this feature to ensure the clinical monitoring team can see their reasoning and thought processes. Oftentimes, CRIO’s built-in alerts are the trigger that ultimately prompts the coordinator to record a note. An unexpected value could lead to a suggestion of documenting an explanation.
By contrast, a traditional eCRF is a secondary data set that requires constant vigilance and attention to maintain. The eCRF contains only structured data. However, eCRF systems deprive the site from preemptively explaining insights about a procedure, visit, or subject that the sponsor would ask for upon reviewing the eCRF entries. As a result, queries within EDC systems often create redundant work for the site because they address information already addressed by the site in source. These queries can be extremely frustrating and demoralizing for sites. Sponsors should understand that these queries represent two extra workflows – one on the part of Data Management in issuing them, and one on the part of the site in answering them.
SIDEBAR: SITE EXPERIENCE
EDC systems limit the ability of Data Management to have direct access to study source material. As a result, I would often receive queries for information that had already been addressed within the source. Sometimes, I was addressing queries from both the data management and monitoring teams on the same data point, and the two teams were completely disconnected from each other.
For instance, I would often have to address pathology or lab results, out of window procedures, out of window visits, non compliant medication adherence, specific investigator interpretations of results, and more, that were already documented and explained within my site’s source. This resulted in a lot of redundant work on my part, and none of this work would have been necessary if the data management team had access to the progress notes our team took the time to write.
John Vatkevich, CCRP (former Hematology and Oncology Site Coordinator of 8 years and current CRIO Employee)
What about the risk of PHI? The good news is that PHI is very rare.
One risk of a progress note within the CRIO system, is if the study team inadvertently inserts Protected Health Information. To be clear, inclusion of PHI in an eSource system is not technically a violation of the patient’s privacy. The study’s informed consent would have contained disclosure that the study sponsors and their representatives have access to the patient’s PHI. However, it is not best practice.
Fortunately, PHI in progress notes is relatively rare. We did an analysis of the progress notes saved by client sites in CRIO, and found only 5 progress notes containing PHI out of a sample of 1000. With further analysis, we estimate that 95% of similarly generated samples will have 0.054%-0.946% of progress notes containing PHI.¹
In other words, less than 1% of progress notes have PHI. This incidence is small enough that it can be managed through targeted site training. It’s a small cost compared to the significant value of enabling full and transparent access to site progress notes.
Final Thoughts
Clinical trials are complex. Accordingly, they always require a certain level of clinical judgment by the investigator. The progress notes tell the “story” behind the PI oversight. Altogether, progress notes are essential for sponsors to review as part of their obligation to perform oversight of quality. CRIO eSource enables real-time application of progress notes to any data point. This offers a significantly more comprehensive and rich data source for studies compared to the eCRF.
Related Reading: eSource makes the eCRF reliable, accurate and timely
_________________________________________________________
¹Methodology: We extracted all 145,771 notes from 1 JAN 2023 through 20 APR 2023 within the U.S. client base. In this analysis, we assume each progress note is independent from another. We generated a random sample of 1,000 and had a trained employee review each note to flag PHI, using the 18 PHI identifiers used in HIPAA. We found 5 that had PHI – all names. There were no addresses, emails or phone numbers. The confidence interval was calculated by Clopper-Pearson intervals with the maximum allowable sample of 1,000. This could not be approximated by a standard normal distribution since the sample of progress notes containing PHI was so small.





